


日用化学工业(中英文) ›› 2024, Vol. 54 ›› Issue (4): 457-466.doi: 10.3969/j.issn.2097-2806.2024.04.012
收稿日期:2023-04-18
修回日期:2024-04-08
出版日期:2024-04-22
发布日期:2024-04-26
基金资助:
Zilong Liu1,2,*( ),Yanxiao Hei1,Di Shi1,Yufei Xiao1,Xue Li1
),Yanxiao Hei1,Di Shi1,Yufei Xiao1,Xue Li1
Received:2023-04-18
Revised:2024-04-08
Online:2024-04-22
Published:2024-04-26
Contact:
*E-mail: 摘要:
提高原油采收率是实现我国能源稳产增产的重要保障,其中以表面活性剂为主剂的化学驱油技术是一种潜力巨大的提高采收率方法。从驱油用表面活性剂的分类出发,详细介绍了不同种类表面活性剂的物化特征及其驱油特性的优缺点,主要包括广泛使用的阴离子、两性和非离子表面活性剂以及新兴的双子表面活性剂等。随着石油行业需求的不断变化,低成本、高效能、绿色的新型表面活性剂已成为未来发展的主要趋势。在驱油过程中表面活性剂的吸附损耗使得溶液中表面活性剂的有效浓度下降,导致驱油效率大大降低。因此,进一步重点综述了驱油用表面活性剂固液界面吸附特性的研究进展,其中吸附等温线模型主要用于评价恒温条件下表面活性剂在固液界面上的吸附量与吸附平衡后表面活性剂浓度的关系,而吸附动力学模型可用于评估吸附速率与时间的关系,揭示表面活性剂吸附的深层机制。为了更佳全面准确地描述表面活性剂吸附特性,有必要联合使用多种吸附模型,也需要发展新型的模型。
中图分类号:
刘子龙, 黑艳晓, 石迪, 肖宇飞, 李雪. 驱油用表面活性剂及其吸附特性的研究进展[J]. 日用化学工业(中英文), 2024, 54(4): 457-466.
Zilong Liu, Yanxiao Hei, Di Shi, Yufei Xiao, Xue Li. Recent advances of surfactants and their adsorption characteristics in oil recovery[J]. China Surfactant Detergent & Cosmetics, 2024, 54(4): 457-466.
表1
常用的阴离子表面活性剂"
| 种类 | 名称 | 分子结构 | 特征 |
|---|---|---|---|
| 磺酸盐 | 烷基磺酸盐 | 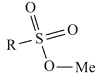 | 在碱性、中性和弱酸性溶液中较为稳定,且耐硬水。烷基磺酸盐的溶解度较大,且随着烷基链的碳数的增长而下降 |
| 烷基苯磺酸盐(ABS) | 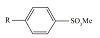 | 在酸性、碱性、硬水及某些氧化物溶液(如次氯酸钠、过氧化物)中都比较稳定,泡沫稳定性较好[ | |
| α-烯烃磺酸盐(AOS) | 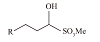 | 耐酸碱、耐盐性高、在冷水中溶解度高且起泡性好 | |
| 内烯烃磺酸盐(IOS) | 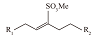 | 双尾、耐高温、增容活性高、成本低等[ | |
| 石油磺酸盐 | 混合物 | 来源广、价格低 | |
| 硫酸盐 | 烷基硫酸盐 | 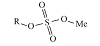 | 良好的乳化性、起泡性、水溶性、可生物降解、耐碱、耐硬水 |
| 烷基苯硫酸盐 | 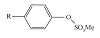 | 可以使磺化反应进行得较完全,副反应少 | |
| 醇烷氧基硫酸盐(AAS) | 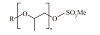 | 耐盐性好,成本低廉 | |
| Guerbet烷氧基硫酸盐(GAS) | 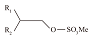 | 耐盐性好,耐高温能力差,在60 ℃以上容易分解 | |
| 羧酸盐 | 醇醚羧酸盐(AEC) | 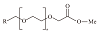 | 羧酸基和烷氧基团的存在使AEC在高温下具有稳定性和耐盐性 |
表2
吸附等温线模型"
| 吸附等温线模型 | 表达式 | 应用假设 |
|---|---|---|
| Henry | Henry吸附等温线假定了溶质的吸附量与相应的平衡溶质浓度呈线性关系[ | |
| Langmuir | Langmuir吸附等温线假设固体表面的同质性,也就是在恒温下发生在均质固体表面的吸附过程[ | |
| Freundlich | qe=KFc1/n | Freundlich吸附等温线用于评估非均质表面上的吸附过程。如果表面活性剂被多层吸附到岩石表面,该等温线可以准确预测表面活性剂的吸附行为[ |
| Temkin | qe=BlnKT+Blnc | Temkin吸附等温线考虑了吸附过程中溶质分子之间的间接相互作用。根据一些实验研究,吸附热在大多数情况下会随着覆盖率的增加而降低。吸附量由结合能的均匀分布来决定,直到达到某个最大结合能[ |
| Redlich-Peterson | Redlich-Peterson吸附等温线由三个不同参数组成的经验等温线,被认为是Langmuir和Freundlich等温线的折中[ |
表3
吸附动力学模型"
| 吸附动力学模型 | 表达式 | 应用假设 |
|---|---|---|
| 拟一级动力学模型(PFO) | 经过长时间的相互作用,吸附量可能远远低于实际平衡吸附量。K的值通常与表面活性剂的初始浓度值成反比。对于许多吸附过程而言,PFO模型通常仅仅适用于吸附开始20~30 min内的早期相互作用过程,并不适用于整个吸附过程[ | |
| 拟二级动力学模型(PSO) | PSO模型适合溶质浓度较低的情况,而PFO模型适用于溶质浓度较高的情况。在大多数情况下,PSO模型同样适用于PFO,具有广泛的适用性,用PSO得到的qe与实验值接近,相关性也更高[ | |
| 粒子内扩散动力学模型(IPD) | qt=Kit1/2+c | Ki一般随着初始吸附质的浓度的增加而增加。如果曲线通过原点(截距为0),IPD控制吸附过程。然而,它有时在整个吸附过程中表现出多重线性。多重线性表示存在多重吸附机制,如传质、吸附模扩散、表面扩散和孔扩散[ |
| Elovich | Elovich方程忽略了化学吸附导致的解吸行为,因为在长时间的吸附过程中,无穷大的qt是不合理的。因此,当系统处于极端不平衡状态时,Elovich模型的应用范围仅限于初始吸附过程[ | |
| Avrami | qt=qe-qeexp(-Ktn) | 在吸附过程中,吸附速率系数可能具有时间依赖性[ |
| [1] |
Sheng J J. Status of surfactant EOR technology[J]. Petroleum, 2015, 1 (2) : 97-105.
doi: 10.1016/j.petlm.2015.07.003 |
| [2] |
Belhaj A F, Elraies K A, Mahmood S M, et al. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review[J]. Journal of Petroleum Exploration and Production Technology, 2020, 10: 125-137.
doi: 10.1007/s13202-019-0685-y |
| [3] |
Saha R, Uppaluri R V S, Tiwari P. Effect of mineralogy on the adsorption characteristics of surfactant: reservoir rock system[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 531: 121-132.
doi: 10.1016/j.colsurfa.2017.07.039 |
| [4] | Han F, Liu W, Guan B, et al. Study on compound oil displacing agent of heavy alkyl benzene sulfonate with ultra-lowinterfacial tension[J]. Applied Chemical Industry, 2021, 50 (9) : 2338-2343, 2347. |
| [5] | Li Y, Zhang W, Shen Z, et al. Pilot test of surfactant-polymer flood with mixtures of anionic-cationic surfactants for high temperature low permeability sandstone reservoir[J]. SPE Reservoir Evaluation & Engineering, 2021, 24 (4) : 889-900. |
| [6] |
Dai H, Xi K, Liu X, et al. Cationic surfactant-based electrolyte additives for uniform lithium deposition via lithiophobic repulsion mechanisms[J]. Journal of the American Chemical Society, 2018, 140 (50) : 17515-17521.
doi: 10.1021/jacs.8b08963 pmid: 30486645 |
| [7] | Song C, Liu X, Chen H. Preparation and characterization of montmorillonite modified by cationic Gemini surfactant[J]. Applied Chemical Industry, 2010, 39 (8) : 1198-1200, 1205. |
| [8] |
Nourafkan E, Asachi M, Hu Z, et al. Synthesis of stable nanoparticles at harsh environment using the synergistic effect of surfactants blend[J]. Journal of Industrial and Engineering Chemistry, 2018, 64: 390-401.
doi: 10.1016/j.jiec.2018.04.002 |
| [9] |
Verma A, Chauhan G, Ojha K. Characterization of α-olefin sulfonate foam in presence of cosurfactants: Stability, foamability and drainage kinetic study[J]. Journal of Molecular Liquids, 2018, 264: 458-469.
doi: 10.1016/j.molliq.2018.05.061 |
| [10] |
Jia J, Li J, Liang Y, et al. Molecular dynamics study on performance of olefin sulfonate at the decane: water interface: effect of molecular architecture[J]. Fuel, 2022, 308: 122013.
doi: 10.1016/j.fuel.2021.122013 |
| [11] |
Wang S, Luan H, Liang X, et al. Recognition and characterization of active fractions from petroleum sulfonate[J]. Journal of Petroleum Science and Engineering, 2020, 187: 106797.
doi: 10.1016/j.petrol.2019.106797 |
| [12] | Yin D, Zhong Y. Performance of SB-16/SDS blend surfactant microemulsion flooding system[J]. China Surfactant Detergent & Cosmetics, 2018, 1: 14-17. |
| [13] |
Puerto M, Hirasaki G J, Miller C A, et al. Surfactant systems for EOR in high-temperature, high-salinity environments[J]. SPE Journal, 2012, 17 (1) : 11-19.
doi: 10.2118/129675-PA |
| [14] |
Lu J, Britton C, Solairaj S, et al. Novel large-hydrophobe alkoxy carboxylate surfactants for enhanced oil recovery[J]. SPE Journal, 2014, 19 (6) : 1024-1034.
doi: 10.2118/154261-PA |
| [15] |
Kumar A, Mandal A. Synthesis and physiochemical characterization of zwitterionic surfactant for application in enhanced oil recovery[J]. Journal of Molecular Liquids, 2017, 243: 61-71.
doi: 10.1016/j.molliq.2017.08.032 |
| [16] |
Gao S, Song Z, Lan F, et al. Studies on physicochemical properties and aggregation behavior of two pairs of betaine surfactants[J]. Industrial & Engineering Chemistry Research, 2019, 58 (49) : 22260-22272.
doi: 10.1021/acs.iecr.9b04593 |
| [17] | Hu Y, Jiao T, Huo Y, et al. Performance of a kind of nonionic ethoxylated surfactants[J]. Applied Chemical Industry, 2021, 50 (7) : 1812-1815. |
| [18] |
Seiedi O, Rahbar M, Nabipour M, et al. Atomic force microscopy (AFM) investigation on the surfactant wettability alteration mechanism of aged mica mineral surfaces[J]. Energy & Fuels, 2011, 25 (1) : 183-188.
doi: 10.1021/ef100699t |
| [19] |
Pal N, Saxena N, Mandal A. Studies on the physicochemical properties of synthesized tailor-made Gemini surfactants for application in enhanced oil recovery[J]. Journal of Molecular Liquids, 2018, 258: 211-224.
doi: 10.1016/j.molliq.2018.03.037 |
| [20] |
Mpelwa M, Tang S, Jin L, et al. The study on the properties of the newly extended Gemini surfactants and their application potentials in the petroleum industry[J]. Journal of Petroleum Science and Engineering, 2020, 186: 106799.
doi: 10.1016/j.petrol.2019.106799 |
| [21] | Yin J, Chen Y, Jiang J, et al. Synthesis and properties of pH and redox dual-switchable surfactant[J]. Chemical Journal of Chinese Universities-Chinese, 2017, 38 (9) : 1645-1653. |
| [22] |
Salager J L, Forgiarini A M, Rondón M J. How to attain ultralow interfacial tension and three-phase behavior with a surfactant formulation for enhanced oil recovery: a review—Part 3. practical procedures to optimize the laboratory research according to the current state of the art in surfactant mixing[J]. Journal of Surfactants and Detergents, 2017, 20: 3-19.
doi: 10.1007/s11743-016-1883-y |
| [23] |
Lu P, He S, Zhou Y, et al. Oxidation-induced breakage of the imine bond and aggregate transition in a Se-containing dynamic covalent surfactant[J]. Langmuir, 2021, 37 (8) : 2833-2842.
doi: 10.1021/acs.langmuir.0c03609 pmid: 33615789 |
| [24] |
Chen A, Chen J, Wang D, et al. CO2/N2-responsive oil-in-water emulsions using a novel switchable surfactant[J]. Journal of Colloid and Interface Science, 2020, 571: 134-141.
doi: S0021-9797(20)30327-1 pmid: 32199266 |
| [25] | Hu R, Tang S, Mpelwa M, et al. Research progress of viscoelastic surfactants for enhanced oil recovery[J]. Energy Exploration & Exploitation, 2021, 39 (4) : 1324-1348. |
| [26] |
Zhu J, Yang Z, Li X, et al. Experimental study on the microscopic characteristics of foams stabilized by viscoelastic surfactant and nanoparticles[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 572: 88-96.
doi: 10.1016/j.colsurfa.2019.03.087 |
| [27] | Li X, Zhang C, Zhang G, et al. Applications of viscoelastic surfactant in oilfield[J]. China Surfactant Detergent & Cosmetics, 2014, 44 (9) : 521-524. |
| [28] | Hussain S M S, Adewunmi A A, Mahboob A, et al. Fluorinated surfactants: A review on recent progress on synthesis and oilfield applications[J]. Advances in Colloid and Interface Science, 2022: 102634. |
| [29] |
Al-Amodi A O, Al-Mubaiyedh U A, Sultan A S, et al. Novel fluorinated surfactants for enhanced oil recovery in carbonate reservoirs[J]. The Canadian Journal of Chemical Engineering, 2016, 94 (3) : 454-460.
doi: 10.1002/cjce.v94.3 |
| [30] | Li Y, Wang Y, Tang L, et al. Synthesis and evaluation of novel gas-wetting reversal agent for shale gas reservoir[C]// SPE Asia Pacific Oil and Gas Conference and Exhibition. SPE, 2018: D022S005R002. |
| [31] |
Saha R, Uppaluri R V S, Tiwari P. Effect of mineralogy on the adsorption characteristics of surfactant: Reservoir rock system[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 531: 121-132.
doi: 10.1016/j.colsurfa.2017.07.039 |
| [32] |
Negin C, Ali S, Xie Q. Most common surfactants employed in chemical enhanced oil recovery[J]. Petroleum, 2017, 3 (2) : 197-211.
doi: 10.1016/j.petlm.2016.11.007 |
| [33] |
Ahmadi M A, Shadizadeh S R. Experimental investigation of a natural surfactant adsorption on shale-sandstone reservoir rocks: Static and dynamic conditions[J]. Fuel, 2015, 159: 15-26.
doi: 10.1016/j.fuel.2015.06.035 |
| [34] | Abu-Alsoud G F, Hawboldt K A, Bottaro C S. Comparison of four adsorption isotherm models for characterizing molecular recognition of individual phenolic compounds in porous tailor-made molecularly imprinted polymer films[J]. ACS Applied Materials & Interfaces, 2020, 12 (10) : 11998-12009. |
| [35] |
Gao M, Xu D, Gao Y, et al. Mussel-inspired triple bionic adsorbent: Facile preparation of layered double hydroxide@polydopamine@metal-polyphenol networks and their selective adsorption of dyes in single and binary systems[J]. Journal of Hazardous Materials, 2021, 420: 126609.
doi: 10.1016/j.jhazmat.2021.126609 |
| [36] | Said K A M, Ismail N Z, Jama’in R L, et al. Application of Freundlich and Temkin isotherm to study the removal of Pb (Ⅱ) via adsorption on activated carbon equipped polysulfone membrane[J]. Int. J. Eng. Technol, 2018, 7 (18) : 91-93. |
| [37] |
Chang Z, Chen X, Peng Y. The adsorption behavior of surfactants on mineral surfaces in the presence of electrolytes: A critical review[J]. Minerals Engineering, 2018, 121: 66-76.
doi: 10.1016/j.mineng.2018.03.002 |
| [38] |
Nworie F S, Nwabue F I, Oti W, et al. Removal of methylene blue from aqueous solution using activated rice husk biochar: Adsorption isotherms, kinetics and error analysis[J]. Journal of the Chilean Chemical Society, 2019, 64 (1) : 4365-4376.
doi: 10.4067/s0717-97072019000104365 |
| [39] |
Lebouachera S E I, Chemini R, Khodja M, et al. Experimental investigations of SDS adsorption on the Algerian rock reservoir: chemical enhanced oil recovery case[J]. Research on Chemical Intermediates, 2018, 44: 7665-7690.
doi: 10.1007/s11164-018-3580-0 |
| [40] | Wang Y H, Han F, Xu F Y, et al. Study on adsorption loss of surfactant flooding system in low permeability reservoir[J]. Fine Petrochemical Industry, 2021, 38 (5) : 54-57. |
| [41] |
Liu Z, Hedayati P, Sudhölter E J R, et al. Adsorption behavior of anionic surfactants to silica surfaces in the presence of calcium ion and polystyrene sulfonate[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 602: 125074.
doi: 10.1016/j.colsurfa.2020.125074 |
| [42] | AkankshaKalra H P, Hui C W, Mackey H, et al. Adsorption of dyes from water on to bamboo-based activated carbon-error analysis method for accurate isotherm parameter determination[J]. Journal of Water Science and Engineering, 2019, 1 (1) : 1-11. |
| [43] | Wan J, Yang Y, Liu X, et al. Adsorption of anionic-nonionic surfactants on Lou soil[J]. Journal of Agro-Environment Science, 2011, 30 (4) : 690-696. |
| [44] |
Ahmadi M A, Shadizadeh S R. Induced effect of adding nano silica on adsorption of a natural surfactant onto sandstone rock: experimental and theoretical study[J]. Journal of Petroleum Science and Engineering, 2013, 112: 239-247.
doi: 10.1016/j.petrol.2013.11.010 |
| [45] | Deng S, Li X, Bai W, et al. Synergistic effect on inhibition by surfactants for steel in hydrochloric acid solution[J]. Cleaning World, 2007 (7) : 1-5. |
| [46] |
de Araújo J D C, de Oliveira G V B, de Meneses Lourenço M C, et al. Adsorption study of non-ionic ethoxylated nonylphenol surfactant for sandstone reservoirs: Batch and continuous flow systems[J]. Journal of Molecular Liquids, 2022, 366: 120313.
doi: 10.1016/j.molliq.2022.120313 |
| [47] |
Saxena N, Kumar A, Mandal A. Adsorption analysis of natural anionic surfactant for enhanced oil recovery: The role of mineralogy, salinity, alkalinity and nanoparticles[J]. Journal of Petroleum Science and Engineering, 2019, 173: 1264-1283.
doi: 10.1016/j.petrol.2018.11.002 |
| [48] |
McKay G, Ho Y S, Ng J C Y. Biosorption of copper from waste waters: a review[J]. Separation and Purification Methods, 1999, 28 (1) : 87-125.
doi: 10.1080/03602549909351645 |
| [49] |
Lima E C, Sher F, Guleria A, et al. Is one performing the treatment data of adsorption kinetics correctly?[J]. Journal of Environmental Chemical Engineering, 2021, 9 (2) : 104813.
doi: 10.1016/j.jece.2020.104813 |
| [50] | Kajjumba G W, Emik S, Öngen A, et al. Modelling of adsorption kinetic processes—errors, theory and application[J]. Advanced Sorption Process Applications, 2018: 1-19. |
| [51] |
Liu Z, Zhao G, Brewer M, et al. Comprehensive review on surfactant adsorption on mineral surfaces in chemical enhanced oil recovery[J]. Advances in Colloid and Interface Science, 2021, 294: 102467.
doi: 10.1016/j.cis.2021.102467 |
| [52] |
Issaoui H, Sallem F, Lafaille J, et al. Biosorption of heavy metals from water onto phenolic foams based on tannins and lignin alkaline liquor[J]. International Journal of Environmental Research, 2021, 15 (2) : 369-381.
doi: 10.1007/s41742-021-00313-5 |
| [53] | Lagergren S. Zur theorie der sogenannten adsorption geloster stoffe[J]. Kungliga Svenska Vetenskapsakademiens Handlingar, 1898, 24: 1-39. |
| [54] |
Barati A, Najafi A, Daryasafar A, et al. Adsorption of a new nonionic surfactant on carbonate minerals in enhanced oil recovery: experimental and modeling study[J]. Chemical Engineering Research and Design, 2016, 105: 55-63.
doi: 10.1016/j.cherd.2015.10.047 |
| [55] |
Saxena N, Kumar S, Mandal A. Adsorption characteristics and kinetics of synthesized anionic surfactant and polymeric surfactant on sand surface for application in enhanced oil recovery[J]. Asia-Pacific Journal of Chemical Engineering, 2018, 13 (4) : e2211.
doi: 10.1002/apj.v13.4 |
| [56] |
Hernández-Barreto D F, Giraldo L, Moreno-Piraján J C. Dataset on adsorption of phenol onto activated carbons: Equilibrium, kinetics and mechanism of adsorption[J]. Data in Brief, 2020, 32: 106312.
doi: 10.1016/j.dib.2020.106312 |
| [57] |
Kou J, Xu S. In situ kinetics and conformation studies of dodecylamine adsorption onto zinc sulfide using a quartz crystal microbalance with dissipation (QCM-D)[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 490: 110-120.
doi: 10.1016/j.colsurfa.2015.11.042 |
| [58] | Shang X, Pu W, Xiao T. Measuring static adsorption of sodium dodecyl sulfate on quartz sand by ultraviolet spectrophotometer[J]. Applied Chemical Industry, 2015, 44 (1) : 101-103, 108. |
| [59] |
Cestari A R, Vieira E F S, Pinto A A, et al. Multistep adsorption of anionic dyes on silica/chitosan hybrid: 1. Comparative kinetic data from liquid-and solid-phase models[J]. Journal of Colloid and Interface Science, 2005, 292 (2) : 363-372.
doi: 10.1016/j.jcis.2005.05.096 |
| [60] |
Tabor R F, Eastoe J, Dowding P J. A two-step model for surfactant adsorption at solid surfaces[J]. Journal of Colloid and Interface Science, 2010, 346 (2) : 424-428.
doi: 10.1016/j.jcis.2010.03.047 pmid: 20385388 |
| [1] | 杨许召,袁康康,吴龙焕,牛彩奇,张盈盈,李亚坤,王军. 表面活性剂在MOFs材料制备中的应用[J]. 日用化学工业(中英文), 2024, 54(5): 581-587. |
| [2] | 何一凡, 吴文海, 苏牧楠, 蒋晓龙, 刘宇红. 拉曼光谱研究表面活性剂对皮肤刺激和皮肤防护的体内分子机制[J]. 日用化学工业(中英文), 2024, 54(4): 401-409. |
| [3] | 张志升, 沈产量, 李建勋, 刘延强, 韩薇薇, 董三宝. 甜菜碱/AOS/Gemini季铵盐三元复合型泡排剂的研制与性能评价[J]. 日用化学工业(中英文), 2024, 54(3): 239-249. |
| [4] | 李国峰, 刘凯楠, 莫文龙, 马腾. 页岩油藏渗吸驱油剂体系性能评价[J]. 日用化学工业(中英文), 2024, 54(3): 250-258. |
| [5] | 侯仕达, 王志飞, 王亚魁, 李俊, 姜亚洁, 耿涛. 多阳离子位点季铵盐与AEC复配体系的应用性能研究[J]. 日用化学工业(中英文), 2024, 54(2): 131-138. |
| [6] | 张红梅, 张永民. [芥酰胺苯甲酸][胆碱]离子液体表面活性剂的合成及性能研究[J]. 日用化学工业(中英文), 2024, 54(2): 149-155. |
| [7] | 刘佩, 潘婷, 裴晓梅, 宋冰蕾, 蒋建中, 崔正刚, Bernard P. Binks. 非离子-阴离子Bola型表面活性剂和纳米SiO2颗粒协同稳定的双重响应型O/W乳状液[J]. 日用化学工业(中英文), 2024, 54(1): 1-15. |
| [8] | 艾浩康, 姜亚洁, 王亚魁, 张璐, 耿涛. 硬脂酸酯双子季铵盐的合成及性能研究[J]. 日用化学工业(中英文), 2024, 54(1): 16-23. |
| [9] | 张婉萍, 林延忠, 张倩洁, 张冬梅, 蒋汶. Ca2+介导的月桂酰甲基牛磺酸钠相行为研究[J]. 日用化学工业(中英文), 2024, 54(1): 32-37. |
| [10] | 常世腾, 蔡小军, 郑延成, 刘雪瑾, 易晓, 蒋筑阳. 琥珀酸酯磺酸盐物化特性及其与甜菜碱复配体系界面性能[J]. 日用化学工业(中英文), 2023, 53(9): 989-998. |
| [11] | 徐德荣,连威,熊金钊,康万利. 致密油藏表面活性剂渗吸影响因素研究[J]. 日用化学工业(中英文), 2023, 53(8): 857-864. |
| [12] | 牛奇奇,吕其超,董朝霞,张风帆,王洪勃. 含蠕虫胶束的泡沫体系的性能研究进展[J]. 日用化学工业(中英文), 2023, 53(8): 915-924. |
| [13] | 王佳锐,魏孝承,张春雪,陈昢圳,郑向群,王强. 水环境样品中表面活性剂检测方法研究进展[J]. 日用化学工业(中英文), 2023, 53(8): 925-934. |
| [14] | 强学峰, 张莉, 郑斌, 侯倩倩, 燕坤. 无机盐KCl对离子型表面活性剂泡沫演化规律研究[J]. 日用化学工业(中英文), 2023, 53(7): 733-741. |
| [15] | 邢环宇, 贾丽华, 赵振龙, 杨瑞, 郭祥峰. 含萘酰亚胺和烷基疏水基的新型表面活性剂合成及性能[J]. 日用化学工业(中英文), 2023, 53(7): 742-747. |
|