


China Surfactant Detergent & Cosmetics ›› 2025, Vol. 55 ›› Issue (11): 1361-1377.doi: 10.3969/j.issn.2097-2806.2025.11.001
• Invited paper • Next Articles
Received:2025-09-01
Revised:2025-09-29
Online:2025-11-22
Published:2025-12-22
CLC Number:
Zeyi Wang, Shuli Dong. Nuclear magnetic resonance studies of G-quadruplex[J].China Surfactant Detergent & Cosmetics, 2025, 55(11): 1361-1377.
Tab.1
The comparison of 1H NMR, 11B NMR, 23Na NMR"
| 1H NMR | 11B NMR | 23Na NMR | |
|---|---|---|---|
| Charge distribution | Spherical symmetry | Asymmetry (significant electric quadrupole moment) | Moderate asymmetry (moderate electric quadrupole moment) |
| Relaxion | Dominated by T1 | Enhanced T2 relaxation due to quadrupolar interactions | Enhanced T2 relaxation due to quadrupolar interactions |
| Linewidth | Narrow | Broad | Very broad |
| Detection sensitivity | High | Low | Medium |
| Experimental requirements | Conventional solution-state NMR | Solution-state NMR or solid-state NMR | Solid-state NMR |
| Typical applications | Confirm the formation of G-quadruplex | Confirm the formation of guanosine borate derivatives and analyze the gelation mechanism | Ascertain the binding sites of Na+ |
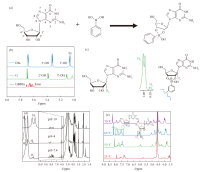
Fig.3
(a) Illustration of the formation of dynamic borate esters via the reaction between guanosine and phenylboronic acid. (b) The 1H NMR of guanosine and GB [38]. (c) Structural formulas of guanosine and GB, along with the ratio of integral areas of H (1'), where Ha represents the H (1') of GB and Hb represents the H (1') of guanosine [40]. (d) 1H NMR spectra of G-quadruplex hydrogels under different pH conditions [40]. (e) 1H NMR spectra of G-quadruplex hydrogels at various temperatures [43]"
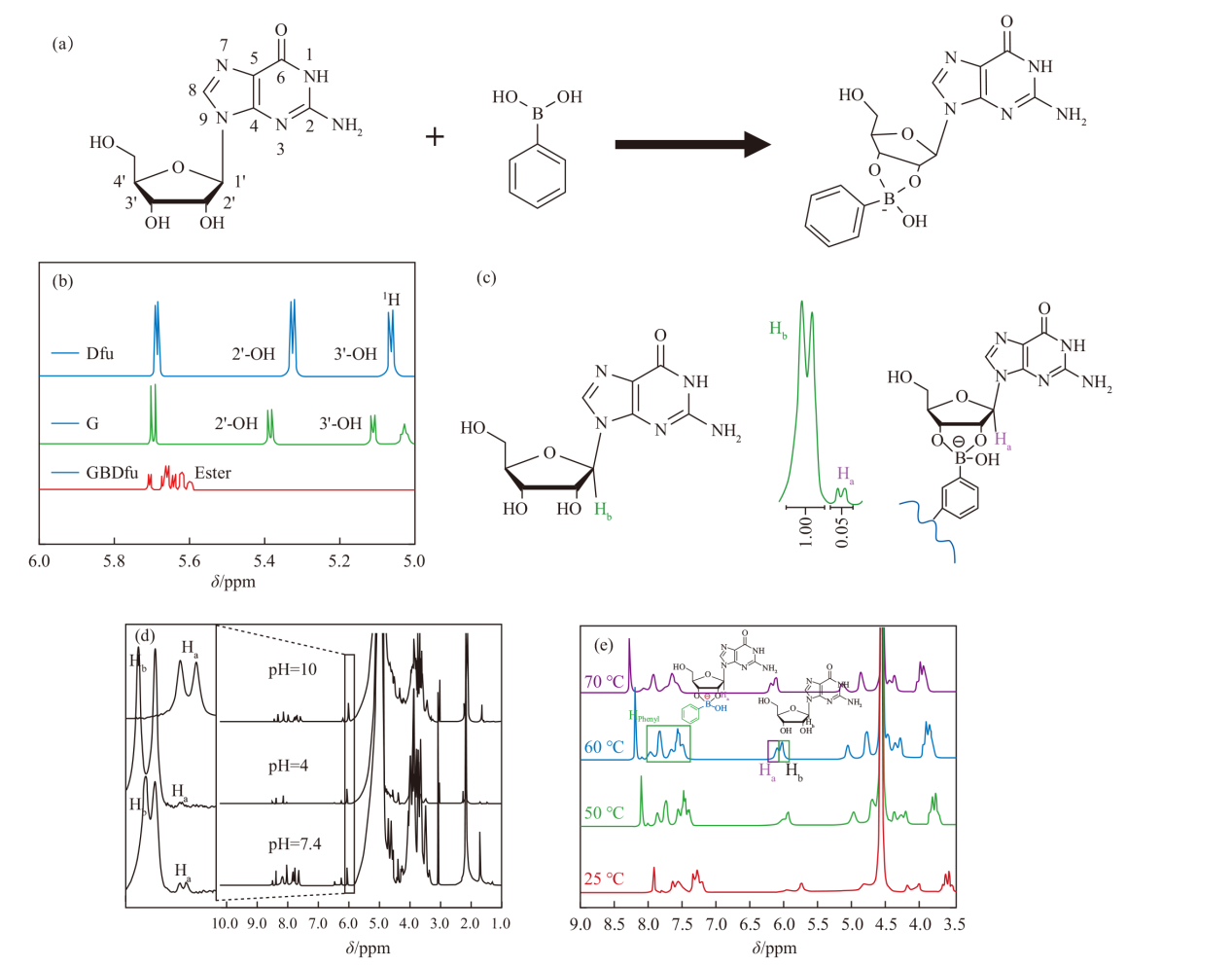
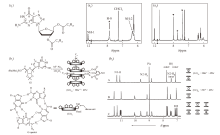
Fig.4
(a1) The structural formula of the lipophilic guanosine derivative 1 and (a2, a3) 1H NMR spectra of the assemblies before and after the addition of K+ in CDCl3, where * represents the H(8) absorption peak of the G-ribbon aggregates, and △ represents the H(8) absorption peak of the G-quadruplex[44]. (b1) Schematic diagram of the G-quadruplex achieving ion-pair recognition, (b2) 1H NMR spectrum of the G-quadruplex in CD2Cl2 [45]"
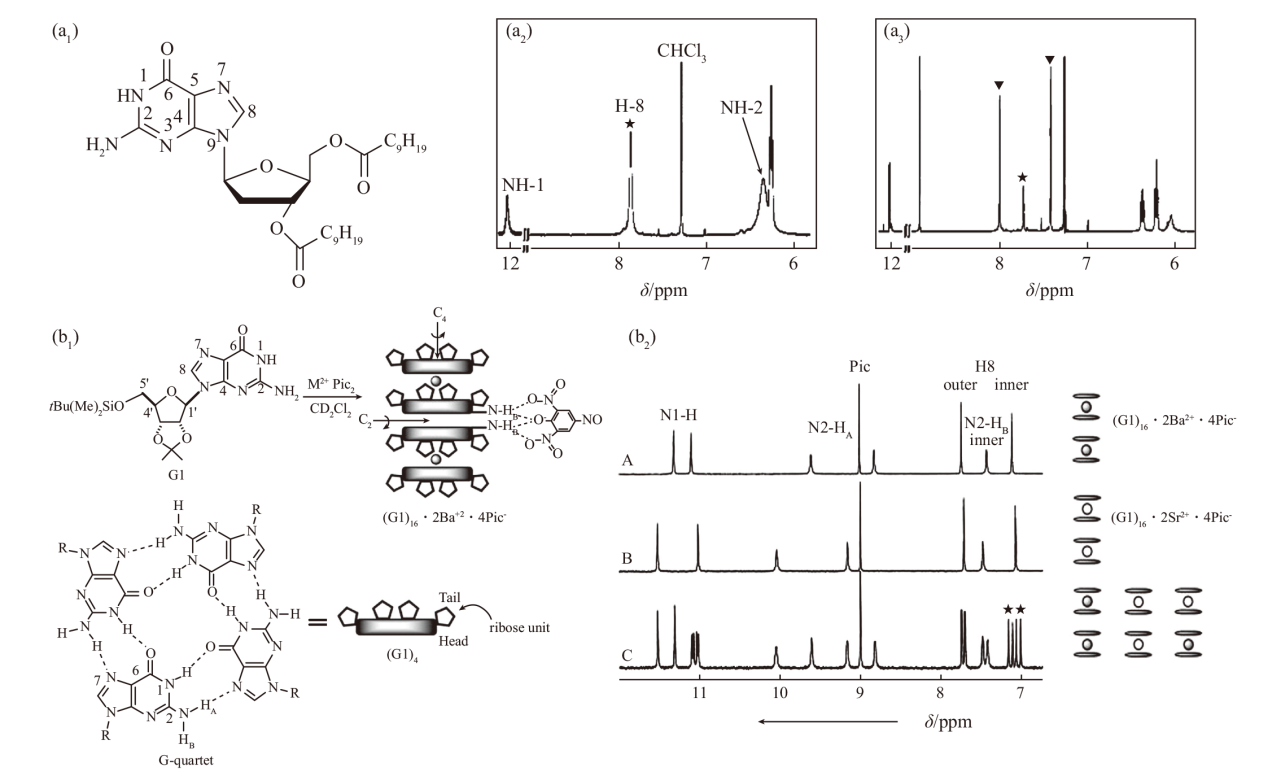

Fig.5
(a1) The structure of guanosine derivative 2. (a2) 1H NMR spectra of TAG and its aggregates [46]. (b) VT-1H NMR spectra of the G-quadruplex formed by isoguanosine [47]. (c) 1H NMR spectra of the aggregates at different temperatures [48]. (d) 1H NMR spectra of the G-quadruplex hydrogel and guanosine [42]. (e) 1H NMR spectra of TAG and the [TAG] 2Ba2+ G-quadruplex [49]"
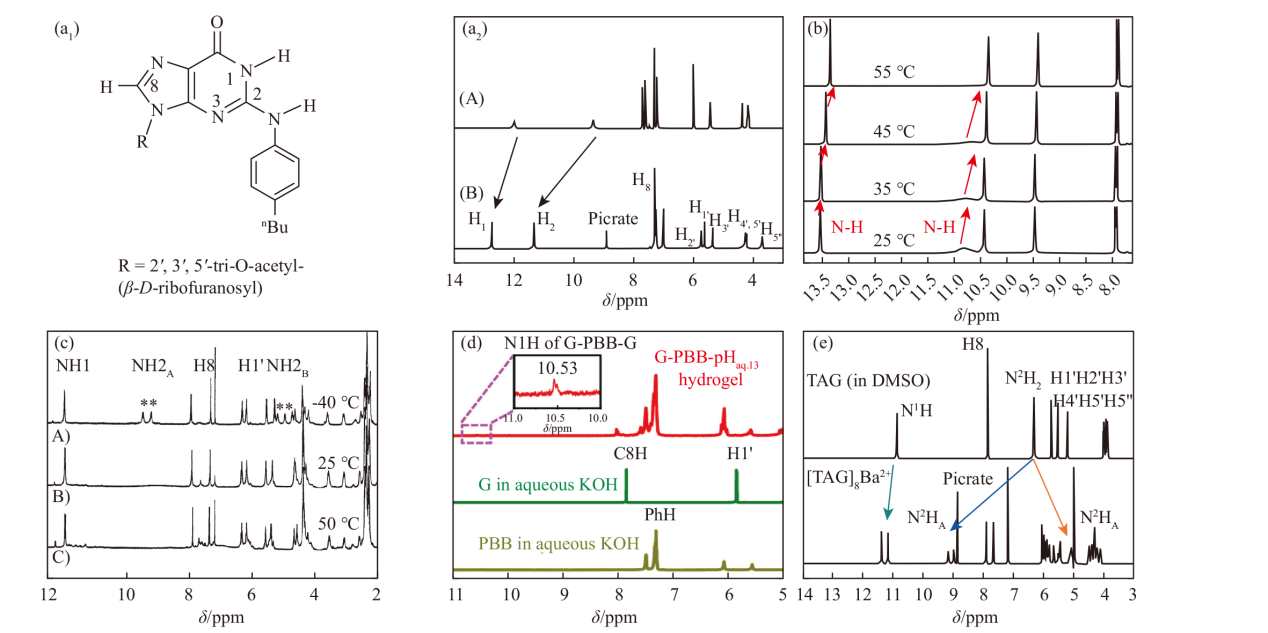
Fig.7
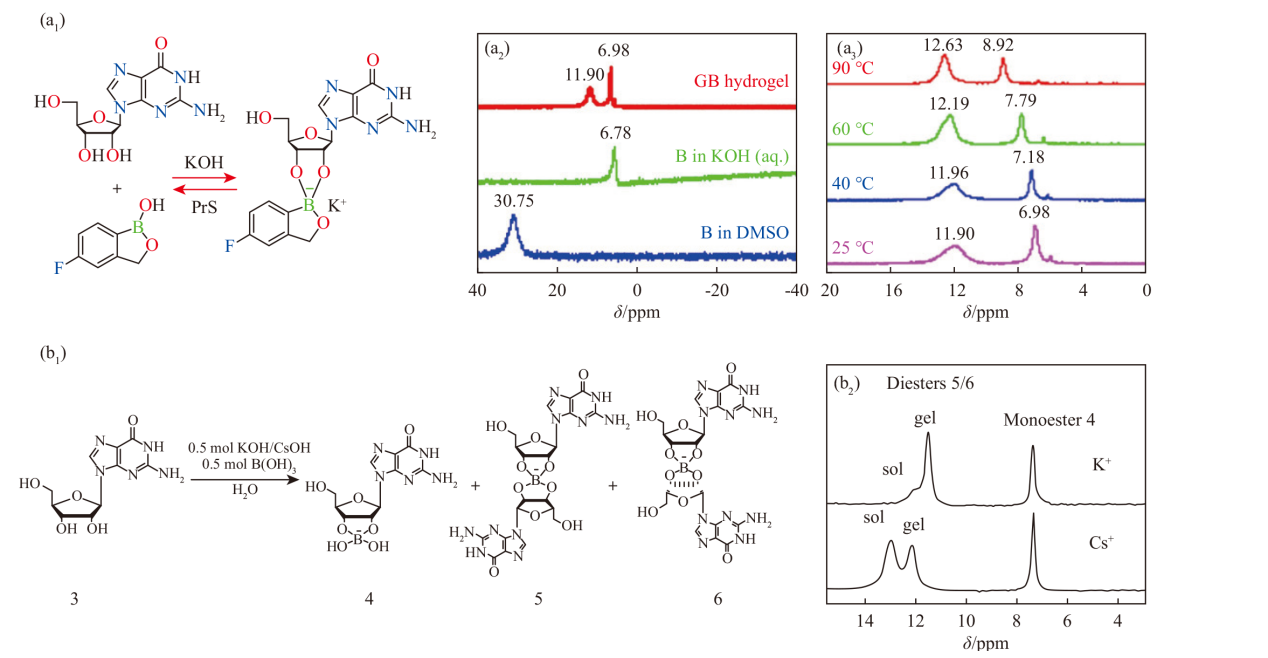
(a1) Schematic illustration of the GB. (a2) 11B NMR of GB (Fig.7a1) dissolved in DMSO, in an alkaline environment, and after assembly with guanosine. (a3) 11B NMR of GB hydrogel at different temperatures [39]. (b1) Proposed mechanism for the reaction between guanosine and KB(OH)4. (b2) 1H-decoupled 11B MAS NMR of K+ GB and Cs+ GB gels [54]"
Tab.2
Comparison of diffusion coefficients determined by NMR and DLS experiments for GMP in H2O [53]"
| NMR experimen | DLS experiment b | |||||
|---|---|---|---|---|---|---|
| w/% | Dt(monomers) c | Dt(quartets) c | Dt(average) | β | w/% | Dt |
| 17.8 | 2.43 | — | — | — | 18 | 3.24±0.09 |
| 24.6 | 1.74 | 0.65 | 1.335 | 0.964 | 25 | 1.14±0.05 |
| 28.9 | 1.45 | 0.58 | 0.983 | 0.956 | 30 | 1.02±0.02 |
| 33.4 | 1.20 | 0.47 | 0.738 | 0.950 | ||
Fig.9
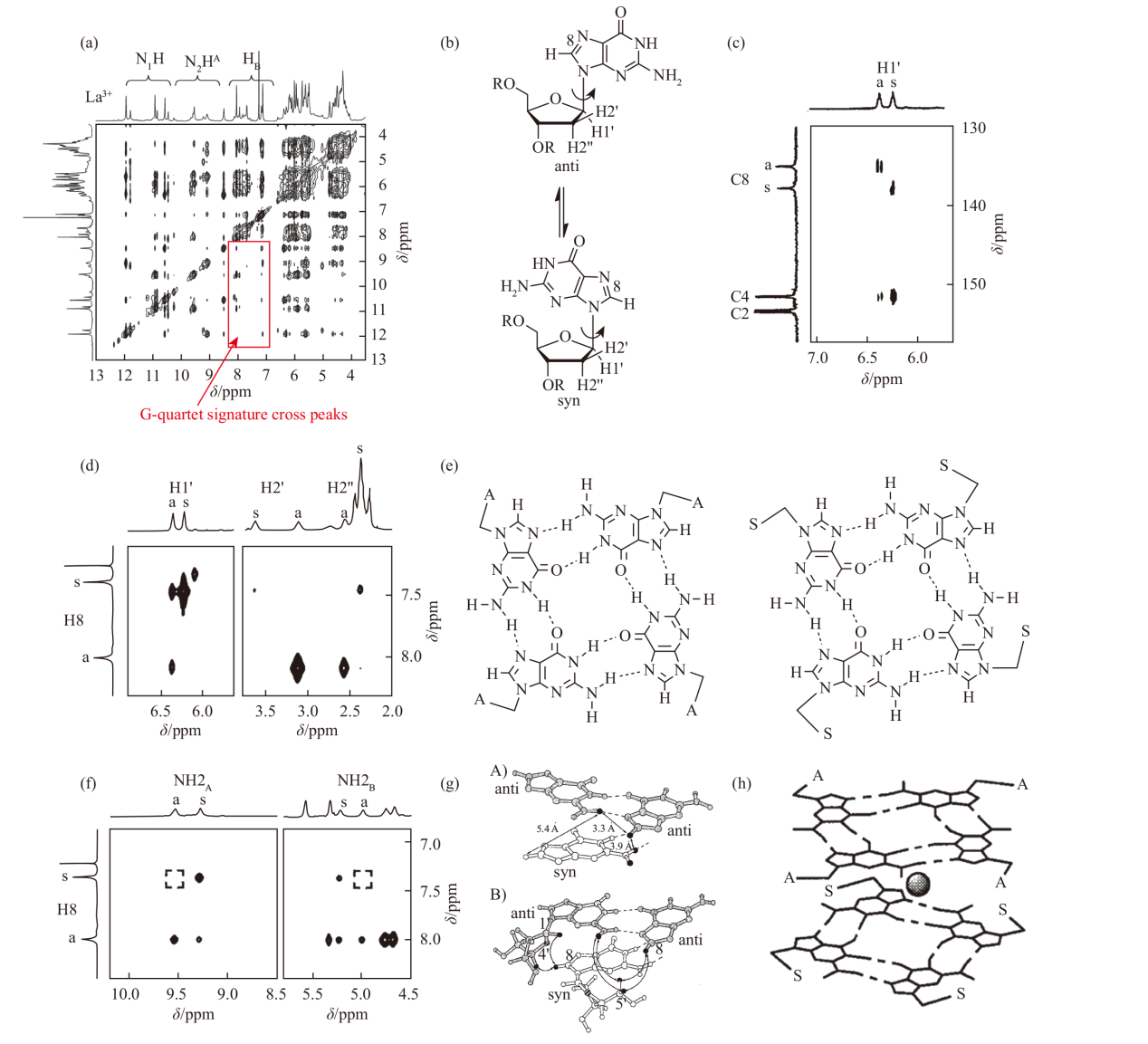
(a) 2D NOESY spectrum of the La3+-G complex [60]. (b) Structures of cis and trans conformers of guanosine derivatives. (c) 13C-1H HMBC spectrum of guanosine derivatives-KI in CDCl3. (d) 2D NOESY spectrum of guanosine derivatives-KI in CDCl3. (e) Schematic of all-cis and all-trans G-quartet assignments. (f) 2D NOESY spectrum of the H(8)-NH2(2) region of guanosine derivatives-KI. (g) Model of guanosine derivatives-KI derived from the 2D NOESY spectrum. (h) Illustration of the head-to-tail stacking mode [48]"
| [1] | Bang I. Über die Guanylsäure[J]. Biological Chemistry, 1910, 69 (2) : 167-168. |
| [2] | Gellert M, Lipsett M N, Davies D R. Helix formation by guanylic acid[J]. Proceedings of the National Academy of Sciences of the United States of America, 1962, 48 (12) : 2013-2018. |
| [3] | Davis J T. G-Quartets 40 years later: From 5′-GMP to molecular biology and supramolecular chemistry Angewandte Chemie International Edition, 2004, 43 (6) : 668-698. |
| [4] | Bhattacharyya D, Mirihana Arachchilage G, Basu S. Metal cations in G-quadruplex folding and stability[J]. Frontiers in Chemistry, 2016, 4: 1-14. |
| [5] |
Ciesielski A, Lena S, Masiero S, et al. Dynamers at the solid-liquid interface: controlling the reversible assembly/reassembly process between two highly ordered supramolecular guanine motifs[J]. Angewandte Chemie International Edition, 2010, 49 (11) : 1963-1966.
doi: 10.1002/anie.v49:11 |
| [6] |
Wang X, Zhou L, Wang H, et al. Reversible organogels triggered by dynamic K+ binding and release[J]. Journal of Colloid and Interface Science, 2011, 353 (2) : 412-419.
doi: 10.1016/j.jcis.2010.09.089 |
| [7] | Rajagopal S K, Hariharan M. Non-natural G-quadruplex in a non-natural environment[J]. Photochemical & Photobiological Sciences, 2014, 13 (2) : 157-161. |
| [8] |
Zhao C, Qu X. Recent progress in G-quadruplex DNA in deep eutectic solvent[J]. Methods, 2013, 64 (1) : 52-58.
doi: 10.1016/j.ymeth.2013.04.017 pmid: 23628945 |
| [9] |
Chen X C, Chen S B, Dai J, et al. Tracking the dynamic folding and unfolding of RNA G-quadruplexes in live cells[J]. Angewandte Chemie International Edition, 2018, 57 (17) : 4702-4706.
doi: 10.1002/anie.v57.17 |
| [10] | Criscuolo A, Napolitano E, Riccardi C, et al. Insights into the small molecule targeting of biologically relevant G-quadruplexes: An overview of NMR and crystal structures[J]. Pharmaceutics, 2022, 14 (11) : 2361. |
| [11] |
Ou T M, Lu Y J, Tan, J H, et al. G-quadruplexes: Targets in anticancer drug design[J]. ChemMedChem, 2008, 3 (5) : 690-713.
doi: 10.1002/cmdc.v3:5 |
| [12] |
Oganesian L, Graham M E, Robinson P J, et al. Telomerase recognizes G-quadruplex and linear DNA as distinct substrates[J]. Biochemistry, 2007, 46 (40) : 11279-11290.
pmid: 17877374 |
| [13] | Alessandrini I, Recagni M, Zaffaroni N, et al. On the road to fight cancer: The potential of G-quadruplex ligands as novel therapeutic agents[J]. International Journal of Molecular Sciences, 2021, 22 (11) : 5947. |
| [14] |
Merlino F, Marzano S, Zizza P, et al. Unlocking the potential of protein-derived peptides to target G-quadruplex DNA: from recognition to anticancer activity[J]. Nucleic Acids Research, 2024, 52 (12) : 6748-6762.
doi: 10.1093/nar/gkae471 pmid: 38828773 |
| [15] | Godoy-Gallardo M, Merino-Gómez M, Matiz L C, et al. Nucleoside-based supramolecular hydrogels: From synthesis and structural properties to biomedical and tissue engineering applications[J]. ACS Biomaterials Science & Engineering, 2023, 9 (1) : 40-61. |
| [16] | Gu C, Xie X Q, Liang Y, et al. Small molecule-based supramolecular-polymer double-network hydrogel electrolytes for ultra-stretchable and waterproof Zn-air batteries working from -50 to 100 ℃[J]. Energy & Environmental Science, 2021, 14 (8) : 4451-4462. |
| [17] |
Thakur N, Chaudhary A, Chakraborty A, et al. Ion conductive phytic acid-G quadruplex hydrogel as electrolyte for flexible electrochromic device[J]. ChemNanoMat, 2021, 7 (6) : 613-619.
doi: 10.1002/cnma.202100072 |
| [18] |
Zhang X, Tang Y, Luo N, et al. Covalently cross-linked supramolecular-polymer dual-network hydrogel with high ionic conductivity and interfacial adhesion for wide temperature-tolerant flexible zinc-air batteries[J]. ACS Applied Polymer Materials, 2024, 6 (9) : 5011-5020.
doi: 10.1021/acsapm.3c03128 |
| [19] | Cho S K, Lee K M, Kang S H, et al. Ion slippage through Li+-centered G-quadruplex[J]. Science Advances, 2022, 8 (37) : eabp8751. |
| [20] |
Kaucher M S, Harrell W A, Davis J T. A unimolecular G-quadruplex that functions as a synthetic transmembrane Na+ transporter[J]. Journal of the American Chemical Society, 2006, 128 (1) : 38-39.
pmid: 16390110 |
| [21] |
Forman S L, Fettinger J C, Pieraccini S, et al. Toward artificial ion channels: A lipophilic G-quadruplex[J]. Journal of the American Chemical Society, 2000, 122 (17) : 4060-4067.
doi: 10.1021/ja9925148 |
| [22] |
Ghosh S, Ghosh T, Bhowmik S, et al. Nucleopeptide-coupled injectable bioconjugated guanosine-quadruplex hydrogel with inherent antibacterial activity[J]. ACS Applied Bio Materials, 2023, 6 (2) : 640-651.
doi: 10.1021/acsabm.2c00912 |
| [23] |
Wu C G, Wang X, Shi Y F, et al. Transforming sustained release into on-demand release: self-healing guanosine-borate supramolecular hydrogels with multiple responsiveness for Acyclovir delivery[J]. Biomaterials Science, 2020, 8 (22) : 6190-6203.
doi: 10.1039/D0BM00966K |
| [24] |
Rit T, Ghosh T, Bhowmik S, et al. Dynamic multicomponent reactions-directed self-assembled G-quadruplex inherent antibacterial hydrogel[J]. Langmuir, 2023, 39 (18) : 6466-6475.
doi: 10.1021/acs.langmuir.3c00392 |
| [25] |
Feng L, Wang H, Liu T, et al. Ultrasensitive and highly selective detection of strontium ions[J]. Nature Sustainability, 2023, 6 (7) : 789-796.
doi: 10.1038/s41893-023-01095-8 |
| [26] |
Sardaru M C, Rosca I, Ursu C, et al. Photothermal hydrogel composites featuring G4-carbon nanomaterial networks for staphylococcus aureus inhibition[J]. ACS Omega, 2024, 9 (14) : 15833-15844.
doi: 10.1021/acsomega.3c07724 |
| [27] |
Chen J, Gao C, Zhang Z, et al. Kinetic control of chirality and circularly polarized luminescence in G-quartet materials[J]. Journal of Materials Chemistry B, 2021, 9 (35) : 7140-7144.
doi: 10.1039/d1tb00683e pmid: 34008691 |
| [28] | Dai Y, Zhang Z, Wang D, et al. Machine-learning-driven G-quartet-based circularly polarized luminescence materials[J]. Advanced Materials, 2024, 36 (4) : e2310455. |
| [29] |
Chen J, Liu X, Suo Z, et al. Right-/left-handed helical G-quartet nanostructures with full-color and energy transfer circularly polarized luminescence[J]. Chemical Communications, 2020, 56 (56) : 7706-7709.
doi: 10.1039/d0cc02449j pmid: 32609116 |
| [30] |
Qi P, Yi M, Song A, et al. Guanine analogue-based assemblies: construction and luminescence functions[J]. Langmuir, 2022, 38 (23) : 7099-7106.
doi: 10.1021/acs.langmuir.2c00705 pmid: 35648843 |
| [31] |
Qi P, Li X, Huang Z, et al. G-quadruplex-based ionogels with controllable chirality for circularly polarized luminescence[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 629: 127411.
doi: 10.1016/j.colsurfa.2021.127411 |
| [32] | Dai Y, Chen J, Zhao C et al. Biomolecule-based circularly polarized luminescent materials: Construction, progress, and applications[J]. Angewandte Chemie International Edition, 2022, 61 (47) : e202211822. |
| [33] |
Yi M, Huang Z, Qi P, et al. Sr2+-Triggered phase transitions from chiral thermotropic liquid crystalline to G-quadruplex CTLC with circularly polarized luminescence[J]. The Journal of Physical Chemistry C, 2021, 125 (35) : 19570-19579.
doi: 10.1021/acs.jpcc.1c06178 |
| [34] |
Adrian M, Heddi B, Phan A T. NMR spectroscopy of G-quadruplexes[J]. Methods, 2012, 57 (1) : 11-24.
doi: 10.1016/j.ymeth.2012.05.003 pmid: 22633887 |
| [35] | Masiero S, Trotta R, Pieraccini S, et al. A non-empirical chromophoric interpretation of CD spectra of DNA G-quadruplex structures[J]. Organic & Biomolecular Chemistry, 2010, 8 (12) : 2683-2692. |
| [36] |
Gray D M, Wen J D, Gray C W, et al. Measured and calculated CD spectra of G-quartets stacked with the same or opposite polarities[J]. Chirality, 2008, 20 (3/4): 431-440.
doi: 10.1002/chir.v20:3/4 |
| [37] |
Liu X, Yu Q, Song A, et al. Progress in nuclear magnetic resonance studies of surfactant systems[J]. Current Opinion in Colloid & Interface Science, 2020, 45: 14-27.
doi: 10.1016/j.cocis.2019.10.006 |
| [38] |
Xia X, Song S, Wen Y, et al. A simple method for fabricating drugs containing acis-o-diol structure into guanosine-based supramolecular hydrogels for drug delivery[J]. Biomaterials Science, 2023, 11 (9) : 3092-3103.
doi: 10.1039/D3BM00057E |
| [39] | Xie X Q, Zhang Y, Liang Y, et al. Programmable transient supramolecular chiral G‐quadruplex hydrogels by a chemically fueled non-equilibrium self‐assembly strategy[J]. Angewandte Chemie International Edition, 2022, 61 (9) : e202114471. |
| [40] |
Sousa V, Amaral A J R, Castanheira E J, et al. Self-supporting hyaluronic acid-functionalized G-quadruplex-based perfusable multicomponent hydrogels embedded in photo-cross-linkable matrices for bioapplications[J]. Biomacromolecules, 2023, 24 (7) : 3380-3396.
doi: 10.1021/acs.biomac.3c00433 |
| [41] | Hu X, Lei S, Song S, et al. Guanosine‐based hydrogel integrating photothermal effect of PDA‐AuNPs through dynamic borate bond for photothermal therapy of cancer[J]. Chemistry-An Asian Journal, 2022, 17 (15) : e202200302. |
| [42] | Li J, Cui Y, Lu Y L, et al. Programmable supramolecular chirality in non-equilibrium systems affording a multistate chiroptical switch[J]. Nature Communications, 2023, 14 (1) : 5030. |
| [43] | Bhattacharyya T, Kumar Y P, Dash J. Supramolecular hydrogel inspired from DNA structures mimics peroxidase activity[J]. ACS Biomaterials Science & Engineering Journal, 2017, 3 (10) : 2358-2365. |
| [44] |
Pieraccini S, Masiero S, Pandoli O, et al. Reversible interconversion between a supramolecular polymer and a discrete octameric species from a guanosine derivative by dynamic cation binding and release[J]. Organic Letters, 2006, 8 (14) : 3125-3128.
pmid: 16805568 |
| [45] |
Shi X, Fettinger J C, Davis J T. Ion-pair recognition by nucleoside self-assembly: guanosine hexadecamers bind cations and anions[J]. Angewandte Chemie International Edition, 2001, 40 (15), 2827-2831.
doi: 10.1002/(ISSN)1521-3773 |
| [46] |
Liu X, Kwan I M, Wang S, et al. G-quartet formation from an N2-modified guanosine derivative[J]. Organic Letters, 2006, 8 (17) : 3685-3688.
pmid: 16898792 |
| [47] |
Zhao H, Feng H, Liu J, et al. Dual-functional guanosine-based hydrogel integrating localized delivery and anticancer activities for cancer therapy[J]. Biomaterials, 2020, 230: 119598.
doi: 10.1016/j.biomaterials.2019.119598 |
| [48] |
Marlow A L, Mezzina E, Spada G P, et al. Cation-templated self-assembly of a lipophilic deoxyguanosine: Solution structure of a K+-dG8 octamer[J]. The Journal of Organic Chemistry, 1999, 64 (14) : 5116-5123.
doi: 10.1021/jo9901440 |
| [49] |
Kwan I C M, She Y, Wu G. Nuclear magnetic resonance and mass spectrometry studies of 2′, 3′, 5′-O-triacetylguanosine self-assembly in the presence of alkaline earth metal ions (Ca2+, Sr2+, Ba2+)[J]. Canadian Journal of Chemistry, 2011, 89 (7) : 835-844.
doi: 10.1139/v10-179 |
| [50] |
Wu G, Kwan I C M. Helical structure of disodium 5′-guanosine monophosphate self-assembly in neutral solution[J]. Journal of the American Chemical Society, 2009, 131 (9) : 3180-3182.
doi: 10.1021/ja809258y |
| [51] |
Panda M, Walmsley J A. Circular dichroism study of supramolecular assemblies of guanosine 5′-monophosphate[J]. The Journal of Physical Chemistry B, 2011, 115 (19) : 6377-6383.
doi: 10.1021/jp201630g |
| [52] |
Peters G M, Skala L P, Plank T N, et al. G4-quartet.M(+) borate hydrogels[J]. Journal of the American Chemical Society, 2015, 137 (17) : 5819-5827.
doi: 10.1021/jacs.5b02753 |
| [53] |
Wong A, Ida R, Spindler L, et al. Disodium guanosine 5'-monophosphate self-associates into nanoscale cylinders at pH 8: A combined diffusion NMR spectroscopy and dynamic light scattering study[J]. Journal of the American Chemical Society, 2005, 127 (19) : 6990-6998.
pmid: 15884942 |
| [54] |
Peters G M, Skala L P, Plank T N. A G4·K+ hydrogel stabilized by an anion[J]. Journal of the American Chemical Society, 2014, 136 (36) : 12596-12599.
doi: 10.1021/ja507506c |
| [55] |
Gu C, Peng Y, Li J, et al. Supramolecular G4 eutectogels of guanosine with solvent-induced chiral inversion and excellent electrochromic activity[J]. Angewandte Chemie International Edition, 2020, 59 (42) : 18768-18773.
doi: 10.1002/anie.v59.42 |
| [56] | Wu G, Wong A. Direct detection of the bound sodium ions in self-assembled 5′-GMP gels: a solid-state 23Na NMR approach[J]. Chemical Communications, 2001, 24: 2658-2659. |
| [57] |
Wong A, Wu G. Selective binding of monovalent cations to the stacking G-quartet structure formed by guanosine 5'-monophosphate: A solid-state NMR study[J]. Journal of the American Chemical Society, 2003, 125 (45) : 13895-13905.
pmid: 14599230 |
| [58] |
Wu G, Wong A, Gan Z, et al. Direct detection of potassium cations bound to G-quadruplex structures by solid-state 39K NMR at 19.6 T[J]. Journal of the American Chemical Society, 2003, 125 (24) : 7182-7183.
doi: 10.1021/ja0340634 |
| [59] |
Hu D, Ren J, Qu X. Metal-mediated fabrication of new functional G-quartet-based supramolecular nanostructure and potential application as controlled drug release system[J]. Chemical Science, 2011, 2 (7) : 1356-1361.
doi: 10.1039/c1sc00109d |
| [60] | Kwan I C M, She Y M, Wu G. Trivalent lanthanide metal ions promote formation of stacking G-quartets[J]. Chemical Communications, 2007, 41: 4286-4288. |
| [61] |
Li X, Huang Z, Li S, et al. A new approach to construct and modulate G-quadruplex by cationic surfactant[J]. Journal of Colloid and Interface Science, 2020, 578: 338-345.
doi: S0021-9797(20)30757-8 pmid: 32535416 |
| [62] |
Reddy G N M, Peters G M, Tatman B P, et al. Magic-angle spinning NMR spectroscopy provides insight into the impact of small molecule uptake by G-quartet hydrogels[J]. Materials Advances, 2020, 1 (7) : 2236-2247.
doi: 10.1039/D0MA00475H |
| [63] |
Yadav R, Patra B, Rai R, et al. Solid-state NMR spectroscopy for unraveling structure and dynamics in biomaterials[J]. Solid State Nuclear Magnetic Resonance, 2025, 140: 102045.
doi: 10.1016/j.ssnmr.2025.102045 |
| [64] |
Wang Z, Qian Y, Qi P, et al. Ionolamellar liquid crystal of G-quadruplex in a protic ionic liquid[J]. Journal of Colloid and Interface Science, 2025, 700: 138479.
doi: 10.1016/j.jcis.2025.138479 |
| [1] | Wanping Zhang, Dexu Meng, Kaikai Liu, Pingli Wang, Qianjie Zhang, Chengliang Li. Assembly behavior and emulsification property of dopamine modified sodium hyaluronate based on ionic coordination [J]. China Surfactant Detergent & Cosmetics, 2025, 55(8): 1006-1016. |
| [2] | Juan Zhang, Ping Liu, Xiaokang Hou, Yuan Gao, Qichao Lv, Zihao Yang. Study on the phase behavior and influencing factors for the emulsions of crude oil/petroleum sulfonate under reservoir conditions [J]. China Surfactant Detergent & Cosmetics, 2025, 55(8): 969-979. |
| [3] | Fengqin Li, Tao Geng, Jingjie Zhou, Jinyuan Sun, Ke Zhang, Chunyu Wang. Study on the mechanism and performance of different catalysts in the reaction between dodecanol and 1, 2-epoxybutane [J]. China Surfactant Detergent & Cosmetics, 2025, 55(8): 998-1005. |
| [4] | Xin Duan, Yanan Zhang, Jiajia Gao, Wanping Zhang, Qianjie Zhang. Study on the phase behavior of mixed systems of cocoyl glycinate surfactants [J]. China Surfactant Detergent & Cosmetics, 2025, 55(7): 861-870. |
| [5] | Linlin Zhao, Yutian Jiao, Li Zhao, Ce Wang, Baocai Xu. Synthesis and self-assembly of an azobenzene-containing dipeptide surfactant [J]. China Surfactant Detergent & Cosmetics, 2024, 54(5): 507-513. |
| [6] | Wanping Zhang, Yanzhong Lin, Qianjie Zhang, Dongmei Zhang, Wen Jiang. Study on the phase behavior of sodium lauroyl methyltaurate mediated by Ca2+ [J]. China Surfactant Detergent & Cosmetics, 2024, 54(1): 32-37. |
| [7] | Dongxia Cao, Lei Zhou, Jiahong Li, Changqin Lin, Jinling Chen. Rapid quantitative analysis of soluble/ionic fluoride in toothpastes [J]. China Surfactant Detergent & Cosmetics, 2024, 54(1): 38-44. |
| [8] | Dou Xin. Analysis of supramolecular structure diversity of gel soft matter system [J]. China Surfactant Detergent & Cosmetics, 2022, 52(9): 945-950. |
| [9] | LI Jun,ZHANG Xing-fang,ZHANG Cheng-wei,XU Na. Mesoscale Brownian dynamics simulation on the self-assembly behaviors of rodlike micelles of CTAC/NaSal surfactants [J]. China Surfactant Detergent & Cosmetics, 2020, 50(4): 213-219. |
| [10] | ZHOU Yuan,YANG Xiu-quan,ZHANG Jun. Performance and phase behavior of the mixture of alkyl polyglycoside sulfosuccinate and alkyl polyglycoside [J]. China Surfactant Detergent & Cosmetics, 2020, 50(1): 20-25. |
| [11] | SUN Na,ZHENG Li-qiang,SUN Ji-chao. Self-assembly of surfactants regulated by weak interactions(IV)Application in ionic conduction [J]. China Surfactant Detergent & Cosmetics, 2019, 49(4): 214-219. |
| [12] | Yang YU,Li-qiang ZHENG,Ji-chao SUN. Self-assembly of surfactants controlled by weak interactions(Ⅲ) Responsive surfactants [J]. China Surfactant Detergent & Cosmetics, 2019, 49(3): 141-149. |
| [13] | Guan-nan SUN,Li-qiang ZHENG,Ji-chao SUN. Self-assembly of surfactants controlled by weak interactions(II)The structure and design of surfactants [J]. China Surfactant Detergent & Cosmetics, 2019, 49(2): 70-75. |
| [14] | WANG Xin-gang,YANG Xiao-yi,SUN Yong-qiang,GUO Chao-hua,LI Ping,LI Jian-bo. Study on the phase behaviors of the mixtures of narrow-distribution sodium fatty alcohol ether sulfate and tetradecyltrimethylammonium bromide [J]. China Surfactant Detergent & Cosmetics, 2019, 49(11): 721-726. |
| [15] | DAI Lu-xun,LIANG Shao-bin,CHEN Yao,XIE Xiang-li,LI Cun-jun,WANG Lin-jiang. Exfoliation of layered double hydroxide and montmorillonite and their self-assembly [J]. China Surfactant Detergent & Cosmetics, 2018, 48(7): 392-398. |
|
