


China Surfactant Detergent & Cosmetics ›› 2026, Vol. 56 ›› Issue (1): 8-16.doi: 10.3969/j.issn.2097-2806.2026.01.002
• Lecture of science and technology • Previous Articles Next Articles
Received:2026-01-21
Online:2026-01-22
Published:2026-02-05
Contact:
E-mail: CLC Number:
Yujun Feng. Viscoelastic surfactants (I)Evolution of micelles and discovery of wormlike micelles[J].China Surfactant Detergent & Cosmetics, 2026, 56(1): 8-16.
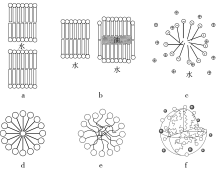
Fig.1
Evolution of the understanding of spherical micelles: (a) Hess lamellar micelle model composed of two McBain lamellar micelles [9]; (b) Harkins cylindrical micelle model showing idealized cross-sections of micelles in the oil-free solubilization state and oil-solubilized state, respectively [10]; (c) Hartley spherical micelle model [14]; (d) Schematic diagram of the “Hartley micelle” that has been misused in textbooks for a long time [15]; (e) Micelle model closer to the actual structure [15]; (f) Schematic diagram of the spherical micelle structure of ionic surfactants [16]"
| [1] | 苏更林. “工业味精”—表面活性剂[J]. 化工之友, 2001(1): 31. |
| [2] | Tadros T F. Applied surfactants: Principles and applications[M]. Wiley: Weinheim, 2005. |
| [3] | 杨锦宗, 张淑芬. 表面活性剂与高新技术产业[J]. 精细化工, 2002, 19(增刊): 1-5. |
| [4] |
Virdi J K, Dusunge A, Handa S. Aqueous micelles as solvent, ligand, and reaction promoter in catalysis[J]. JACS Au, 2024, 4(2): 301-317.
doi: 10.1021/jacsau.3c00605 pmid: 38425936 |
| [5] | McBain J W. General discussion on colloids and their viscosity[J]. Trans. Faraday Soc., 1913, 9: 99-101. |
| [6] | McBain J W. In: Advances in colloid science[M]. Kramer E O, Ed. Interscience Publishers, New York, 1942. |
| [7] | McBain J W. Colloid science[M]. Heath and Company, San Francisco, 1950. |
| [8] |
Hess K, Gundermann J. Roentgen graphical tests on inactive and pouring colloid solutions (Evidence of the orientation from colloid parts in the flow through capillaries through the occurrence of fiber diagrams, hydration of colloid parts in the solution)[J]. Ber. Dtsch. Chem. Ges., 1937, 70: 1800-1808.
doi: 10.1002/cber.v70:8 |
| [9] |
Hess K, Phllippoff W, Kiessig H. Soap solutions[J]. Kolloid Z., 1939, 88: 40-51.
doi: 10.1007/BF01518887 |
| [10] |
Harkins W D, Davies E C H, Clark G L. The orientation of molecules in the surfaces of liquids, the energy relations at surfaces, solubility, adsorption, emulsification, molecular association, and the effect of acids and bases on interfacial tension (Surface energy VI)[J]. J. Am. Chem. Soc., 1917, 39: 541-596.
doi: 10.1021/ja02249a002 |
| [11] |
Harkins W D, Mattoon R W, Corrin M L. Structure of soap micelles indicated by X-rays and the theory of molecular orientation. I. Aqueous solutions[J]. J. Am. Chem. Soc., 1946, 68: 220-228.
doi: 10.1021/ja01206a022 |
| [12] |
Mattoon R W, Stearns R S, Harkins W D. Structure for soap micelles as indicated by a previously unrecognized X-ray diffraction band[J]. J. Chem. Phys., 1947, 15: 209-210.
doi: 10.1063/1.1746475 |
| [13] |
Harkins W D. A cylindrical model for the smallsoap micelle[J]. J. Chem. Phys., 1948, 16: 156-157.
doi: 10.1063/1.1746811 |
| [14] | Hartley G S. Aqueous solutions of paraffinic-chain salts. A study of micelle formation[M]. Herman, Paris, 1936. |
| [15] |
Menger F M, Zana R, Lindman B. Portraying the structure of micelles[J]. J. Chem. Ed., 1998, 75(1): 115.
doi: 10.1021/ed075p115 |
| [16] |
van Stam J, Depaemelaere S, de Schryver F C. Micellar aggregation numbers-a fluorescence study[J]. J. Chem. Ed., 1998, 75(1): 93-98.
doi: 10.1021/ed075p93 |
| [17] |
Debye P. Note on light scattering in soap solutions[J]. J. Colloid Sci., 1948, 3: 407-409.
pmid: 18877004 |
| [18] |
Debye P. Light scattering in soap solutions[J]. J. Phys. Colloid Chem., 1949, 53: 1-8.
pmid: 18112142 |
| [19] |
Debye P. Light scattering in soap solutions[J]. Ann. NY Acad. Sci., 1949, 51: 575-592.
doi: 10.1111/nyas.1949.51.issue-4 |
| [20] |
Poland D C, Scheraga H A. Hydrophobic bonding and micelle stability[J]. J. Phys. Chem., 1965, 69: 2431-2442.
doi: 10.1021/j100891a055 |
| [21] |
Poland D C, Scheraga H A. Hydrophobic bonding and micelle stability: The influence of ionic head groups[J]. J. Colloid Interface Sci., 1966, 21: 273-283.
doi: 10.1016/0095-8522(66)90012-2 |
| [22] | Tanford C. The hydrophobic effect[M]. Wiley, New York, 1973. |
| [23] |
Tanford C. Thermodynamics of micelle formation: Prediction of micelle size and size distribution[J]. Proc. Natl. Acad. Sci. USA, 1974, 71: 1811-1815.
pmid: 4525294 |
| [24] |
Tanford C. Theory of micelle formation in aqueous solutions[J]. J. Phys. Chem., 1974, 78: 2469-2479.
doi: 10.1021/j100617a012 |
| [25] |
Tartar H V. A theory of the structure of the micelles of normal paraffin chain salts in aqueous solution[J]. J. Phys. Chem., 1955, 59: 1195-1199.
doi: 10.1021/j150534a004 |
| [26] |
Israelachvili J N, Mitchell J D, Ninham B W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers[J]. J. Chem. Soc. Faraday Trans. II, 1976, 72: 1525-1568.
doi: 10.1039/f29767201525 |
| [27] |
Czajka A, Hazell G, Eastoe J. Surfactants at the design limit[J]. Langmuir, 2015, 31: 8205-8217.
doi: 10.1021/acs.langmuir.5b00336 pmid: 25797065 |
| [28] |
Zhang Y, Feng Y, Wang Y, et al. CO2‑switchable viscoelastic fluids based on a pseudogemini surfactant[J]. Langmuir, 2013, 29: 4187-4192.
doi: 10.1021/la400051a |
| [29] |
Nagarajan R, Ruckenstein E. Theory of surfactant self-assembly: A predictive molecular thermodynamic approach[J]. Langmuir, 1991, 7: 2934-2969.
doi: 10.1021/la00060a012 |
| [30] | Nagarajan R. One hundred years of micelles:Evolution of the theory of micellization. In: RomstedS,ed. Surfactant Science and Technology[M]. CRC Press, Taylor & Francis, 2014: 3-52. |
| [31] |
Stigter D. Intrinsic viscosity and flexibility of rodlike detergent micelles[J]. J. Phys. Chem. 1966, 70(4): 1323-1325.
doi: 10.1021/j100876a507 |
| [32] |
Rupar P A, Chabanne L, Winnik M A, et al. Non-centrosymmetric cylindrical micelles by unidirectional growth[J]. Science, 2012, 337(6094): 559-562.
doi: 10.1126/science.1221206 pmid: 22859484 |
| [33] |
Shikata T, Shiokawa M, Imai S I. Viscoelastic behavior of surfactant threadlike micellar solutions: effects of additives[J]. J. Colloid Interface Sci., 2003, 259(2): 367-373.
doi: 10.1016/S0021-9797(02)00232-1 |
| [34] | Zana R, Kaler E W. Giant micelles: Properties and applications[M]. CRC Press, Boca Raton, 2007. |
| [35] |
Cates M E, Fielding S M. Rheology of giant micelles[J]. Adv. Phys., 2006, 55(7-8): 799-879.
doi: 10.1080/00018730601082029 |
| [36] |
Philippoff W. Colloidal and polyelectrolytes. The micelle and swollen micelle. On soap micelles[J]. Discuss. Faraday Soc., 1951, 11: 96-107.
doi: 10.1039/df9511100096 |
| [37] |
Debye P, Anacker E W. Micelle shape from dissymmetry measurements[J]. J. Phys. Colloid Chem., 1951, 55: 644-655.
pmid: 14832754 |
| [38] | Anacker E W. In: Cationic surfactants[M]. E. Jungermann, Ed., Chap. 7. Marcel Dekker, New York, 1970. |
| [39] |
Nemethy G, Scheraga H A. Structure of water and hydrophobic bonding in proteins. 3. Thermodynamic properties of hydrophobic bonds in proteins[J]. J. Phys. Chem., 1962, 66: 1773-3417.
doi: 10.1021/j100816a004 |
| [40] |
Pilpel N. On gel formation in soaps[J]. J. Colloid Sci., 1954, 9: 285-299.
doi: 10.1016/0095-8522(54)90039-2 |
| [41] |
Pilpel N. Viscoelasticity in aqueous soap solutions[J]. J. Phys. Chem., 1956, 60: 779-782.
doi: 10.1021/j150540a018 |
| [42] |
Gravsholt S. Viscoelasticity in highly dilute aqueous solutions of pure cationic detergents[J]. J. Colloid Interface Sci., 1976, 57(3): 575-577.
doi: 10.1016/0021-9797(76)90236-8 |
| [43] |
Ulmius J, Wennerström H, Johansson L B Å, et al. Viscoelasticity in surfactant solutions: characteristics of the micellar aggregates and the formation of periodic colloidal structures[J]. J. Phys. Chem., 1979, 83(17): 2232-2236.
doi: 10.1021/j100480a010 |
| [44] |
Appell J, Porte G. An investigation on the micellar shape using angular dissymmetry of light scattered by solutions of cetylpyridinium bromide[J]. J. Colloid Interface Sci., 1981, 81(1): 85-90.
doi: 10.1016/0021-9797(81)90305-2 |
| [45] |
Ikeda S, Hayashi S, Imae T. Rodlike micelles of sodium dodecyl sulfate in concentrated sodium halide solutions[J]. J. Phys. Chem. 1981, 85(1), 106-112.
doi: 10.1021/j150601a024 |
| [46] |
Imae T, Kamiya R, Ikeda S. Formation of spherical and rod-like micelles of cetyltrimethylammonium bromide in aqueous NaBr solutions[J]. J. Colloid Interface Sci., 108(1): 215-225.
doi: 10.1016/0021-9797(85)90253-X |
| [47] | Manohar C, Rao U R K, Valaulikar B S, et al. On the origin of viscoelasticity in micellar solutions of cetyltrimethylammonium bromide and sodium salicylate[J]. J. Chem. Soc., Chem. Commun., 1986(5): 379-381. |
| [48] |
Lin T L, Chem S H, Gabriel N E, et al. Small-angle neutron scattering techniques applied to the study of polydisperse rodlike diheptanoylphosphatidylcholine micelles[J]. J. Phys. Chem. 1987, 91(2): 406-413.
doi: 10.1021/j100286a031 |
| [49] |
Lin Z, Scriven L E, Davis H T. Cryogenic electron microscopy of rodlike or wormlike micelles in aqueous solutions of nonionic surfactant hexaethylene glycol monohexadecyl ether[J]. Langmuir, 1992, 8: 2200-2205.
doi: 10.1021/la00045a021 |
| [50] |
Zana R, Talmon Y. Dependence of aggregate morphology on structure of dimeric surfactants[J]. Nature, 1993, 362: 228-230.
doi: 10.1038/362228a0 |
| [51] |
Danino D, Talmon Y, Levy H, et al. Branched threadlike micelles in an aqueous solution of a trimeric surfactant[J]. Science, 1995, 269(5229): 1420-1421.
pmid: 17731153 |
| [52] |
Cates M E. Dynamics of living polymers and flexible surfactant micelles: scaling laws for dilution[J]. J. Phys. Fr., 1988, 49: 1593-1600.
doi: 10.1051/jphys:019880049090159300 |
| [53] |
Cates M E. Reptation of living polymers-Dynamics of entangled polymers in the presence of reversible chain-scission reactions[J]. Macromolecules, 1987, 20: 2289-2296.
doi: 10.1021/ma00175a038 |
| [54] |
Magid L J. The surfactant-polyelectrolyte analogy[J]. J. Phys. Chem. B, 1998, 102: 4064-4074.
doi: 10.1021/jp9730961 |
| [55] |
Padding J T, Briels W J, Stukan M R, et al. Review of multi-scale particulate simulation of the rheology of wormlike micellar fluids[J]. Soft Matter, 2009, 5: 4367-4375.
doi: 10.1039/b911329k |
| [56] |
Rehage H, Hoffmann H. Rheological properties of viscoelastic surfactant systems[J]. J. Phys. Chem., 1988, 92(16): 4712-4719.
doi: 10.1021/j100327a031 |
| [57] |
Rehage H, Hoffmann H. Viscoelastic surfactant solutions: model systems for rheological research[J]. Mol. Phys., 1991, 74(5): 933-973.
doi: 10.1080/00268979100102721 |
| [58] | Rehage H, Hoffmann H. Viscoelastic surfactant solutions[C]// Herb C A, Prud’homme R K, eds. Structure and Flow in Surfactant Solutions. ACS Symp. Ser. 578, Washington DC, 1994: 22-31. |
| [59] | Yang J. Viscoelastic wormlike micelles and their applications[J]. Curr. Opi. Colloid Interface Sci., 2002, 7(5-6): 276-281. |
| [60] | Chase B, Chmilowski W, Dang Y, et al. Clear fracturing fluids for increased well productivity[J]. Oilfield Rev., 1997(3): 20-33. |
| [61] | 牟建海, 李干佐, 肖洪地, 廖广志, 刘奕, 黄丽, 李伯勤. 阴离子表面活性剂AES虫状胶束的形成[J]. 科学通报, 2001, 46(9): 723-726. |
| [62] |
Chu Z, Dreiss C A, Feng Y. Smart wormlike micelles[J]. Chem. Soc. Rev., 2013, 42(17): 7174-7203.
doi: 10.1039/c3cs35490c pmid: 23545844 |
| [63] | Feng Y, Chu Z, Dreiss C A. Smart wormlike micelles: design, characteristics and applications[J]. Springer, Berlin, 2015. |
| [1] | Wanping Zhang, Dexu Meng, Kaikai Liu, Pingli Wang, Qianjie Zhang, Chengliang Li. Assembly behavior and emulsification property of dopamine modified sodium hyaluronate based on ionic coordination [J]. China Surfactant Detergent & Cosmetics, 2025, 55(8): 1006-1016. |
| [2] | Fengqin Li, Tao Geng, Jingjie Zhou, Jinyuan Sun, Ke Zhang, Chunyu Wang. Study on the mechanism and performance of different catalysts in the reaction between dodecanol and 1, 2-epoxybutane [J]. China Surfactant Detergent & Cosmetics, 2025, 55(8): 998-1005. |
| [3] | Qianjie Zhang, Zhenzhi Zhao, Shanshan Wang, Jie Gu, Zhi Lv, Wanping Zhang. Effect of hydrophobic groups of polyglycerol ester emulsifiers on liquid crystal formation and its promotion mechanism [J]. China Surfactant Detergent & Cosmetics, 2025, 55(12): 1552-1559. |
| [4] | Zeyi Wang, Shuli Dong. Nuclear magnetic resonance studies of G-quadruplex [J]. China Surfactant Detergent & Cosmetics, 2025, 55(11): 1361-1377. |
| [5] | Linlin Zhao, Yutian Jiao, Li Zhao, Ce Wang, Baocai Xu. Synthesis and self-assembly of an azobenzene-containing dipeptide surfactant [J]. China Surfactant Detergent & Cosmetics, 2024, 54(5): 507-513. |
| [6] | Liang Yihuan,Du Jing. “Jelly” phenomenon rheology study and improvement in shampoo system [J]. China Surfactant Detergent & Cosmetics, 2022, 52(9): 920-929. |
| [7] | Dou Xin. Analysis of supramolecular structure diversity of gel soft matter system [J]. China Surfactant Detergent & Cosmetics, 2022, 52(9): 945-950. |
| [8] | LI Jun,ZHANG Xing-fang,ZHANG Cheng-wei,XU Na. Mesoscale Brownian dynamics simulation on the self-assembly behaviors of rodlike micelles of CTAC/NaSal surfactants [J]. China Surfactant Detergent & Cosmetics, 2020, 50(4): 213-219. |
| [9] | SUN Na,ZHENG Li-qiang,SUN Ji-chao. Self-assembly of surfactants regulated by weak interactions(IV)Application in ionic conduction [J]. China Surfactant Detergent & Cosmetics, 2019, 49(4): 214-219. |
| [10] | Yang YU,Li-qiang ZHENG,Ji-chao SUN. Self-assembly of surfactants controlled by weak interactions(Ⅲ) Responsive surfactants [J]. China Surfactant Detergent & Cosmetics, 2019, 49(3): 141-149. |
| [11] | Guan-nan SUN,Li-qiang ZHENG,Ji-chao SUN. Self-assembly of surfactants controlled by weak interactions(II)The structure and design of surfactants [J]. China Surfactant Detergent & Cosmetics, 2019, 49(2): 70-75. |
| [12] | DAI Lu-xun,LIANG Shao-bin,CHEN Yao,XIE Xiang-li,LI Cun-jun,WANG Lin-jiang. Exfoliation of layered double hydroxide and montmorillonite and their self-assembly [J]. China Surfactant Detergent & Cosmetics, 2018, 48(7): 392-398. |
| [13] | CHEN Yu-jia, LIU Jia-xi, ZHU Qian-qian, HU Song-qing, SUN Shuang-qing, WANG Xiu-min. Effects of sodium salicylate on the self-assembly behavior of DTAB/PAM mixed system [J]. China Surfactant Detergent & Cosmetics, 2018, 48(6): 308-313. |
| [14] | REN Shu-jing, ZHENG Li-qiang, SUN Ji-chao. Self-assembly of surfactants regulated by weak interaction (Ⅰ)The classification of weak interactions [J]. China Surfactant Detergent & Cosmetics, 2018, 48(11): 611-616. |
| [15] | ZHANG Meng, QI Li-yun, LONG Yu, WANG Ji-yuan, QI Song-zhu, HUANG Yuan-lin, FANG Xue-jin. Study on rheological behaviors of pH-responsive wormlike micelles formed by an amine oxide surfactant [J]. China Surfactant Detergent & Cosmetics, 2017, 47(6): 301-306. |
|
||
