


China Surfactant Detergent & Cosmetics ›› 2024, Vol. 54 ›› Issue (1): 1-15.doi: 10.3969/j.issn.2097-2806.2024.01.001
• Basic research • Next Articles
Pei Liu1,2,Ting Pan1,Xiaomei Pei1,*( ),Binglei Song1,Jianzhong Jiang1,Zhenggang Cui1,*(
),Binglei Song1,Jianzhong Jiang1,Zhenggang Cui1,*( ),Bernard P. Binks3,*(
),Bernard P. Binks3,*( )
)
Received:2023-08-25
Revised:2023-12-02
Online:2024-01-22
Published:2024-01-26
Contact:
*Tel.: +86-13382888025, E-mail: pxmxiaomei@163.com(Xiaomei Pei); cuizhenggang@hotmail.com(Zhenggang Cui); b.p.binks@hull.ac.uk(Bernard P. Binks).
CLC Number:
Pei Liu, Ting Pan, Xiaomei Pei, Binglei Song, Jianzhong Jiang, Zhenggang Cui, Bernard P. Binks. Dual-responsive oil-in-water emulsions co-stabilized by a nonionic-anionic Bola surfactant and silica nanoparticles[J].China Surfactant Detergent & Cosmetics, 2024, 54(1): 1-15.
Tab. 1
Surface activity parameters of CH3O(EO)5-R11-COOH at different pH and comparison with that of C12EO5 at 25 ℃"
| Surfactant | cmc/(mmol/L) | γcmc/(mN/m) | Γmax/(10-10 mol/cm2) | Amin/(nm2/molec) |
|---|---|---|---|---|
| CH3O(EO) 5-R11-COOH (pH=3.0) | 0.032 | 41.4 | 2.65 | 0.63 |
| CH3O(EO) 5-R11-COONa (pH=7.3) | 0.075 | 42.0 | 2.25 | 0.74 |
| CH3O(EO) 5-R11-COONa (pH=12.0) | 0.350 | 41.5 | 1.70 | 1.01 |
| C12EO5[ | 0.060 | 30.4 | 3.42 | 0.49 |

Fig. 6
(A) Photos of conventional emulsions of n-decane-in-water stabilized by 0.1 mmol/L CH3O(EO) 5-R11-COOH alone at different pH taken 24 h after preparation at 25 ℃. (B and C) Photos and optical micrographs of conventional emulsions of n-decane-in-water (7 mL/7 mL) stabilized solely by CH3O(EO) 5-R11-COONa at pH of 10.9 at different concentrations (given in mmol/L) taken one month after preparation at 25 ℃"


Fig. 7
pH-Responsiveness of conventional emulsions of n-decane-in-water stabilized by (A) 1 mmol/L CH3O(EO) 5-R11-COOH alone and (B) 3 mmol/L R11-COONa alone after addition of 0.2 mol/L HCl (“Off (1)”) or 0.2 mol/L NaOH (“On (1)”) at 25 ℃. After demulsification the separated oil was removed and fresh oil was added before re-homogenization (H)"

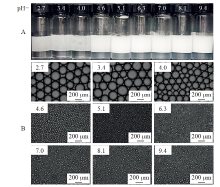
Fig. 8
(A) Photos and (B) optical micrographs of n-decane-in-water (7 mL/7 mL) emulsions co-stabilized by 0.1 mmol/L CH3O(EO)5-R11-COOH and 0.1 wt.% silica nanoparticles at different pH taken 24 h after preparation at 25 ℃. The first six are Pickering emulsions, and the last three are oil-in-dispersion emulsions"


Fig. 11
(A, B) Digital photos and (C) micrographs of n-decane-in-water (7 mL/7 mL) oil-in-dispersion emulsions co-stabilized by CH3O(EO) 5-R11-COONa of different concentration (shown in mmol/L) and 0.1 wt.% silica nanoparticles at pH of 9.0, taken (A) 24 h and (B, C) 7 days after preparation at 25 ℃"
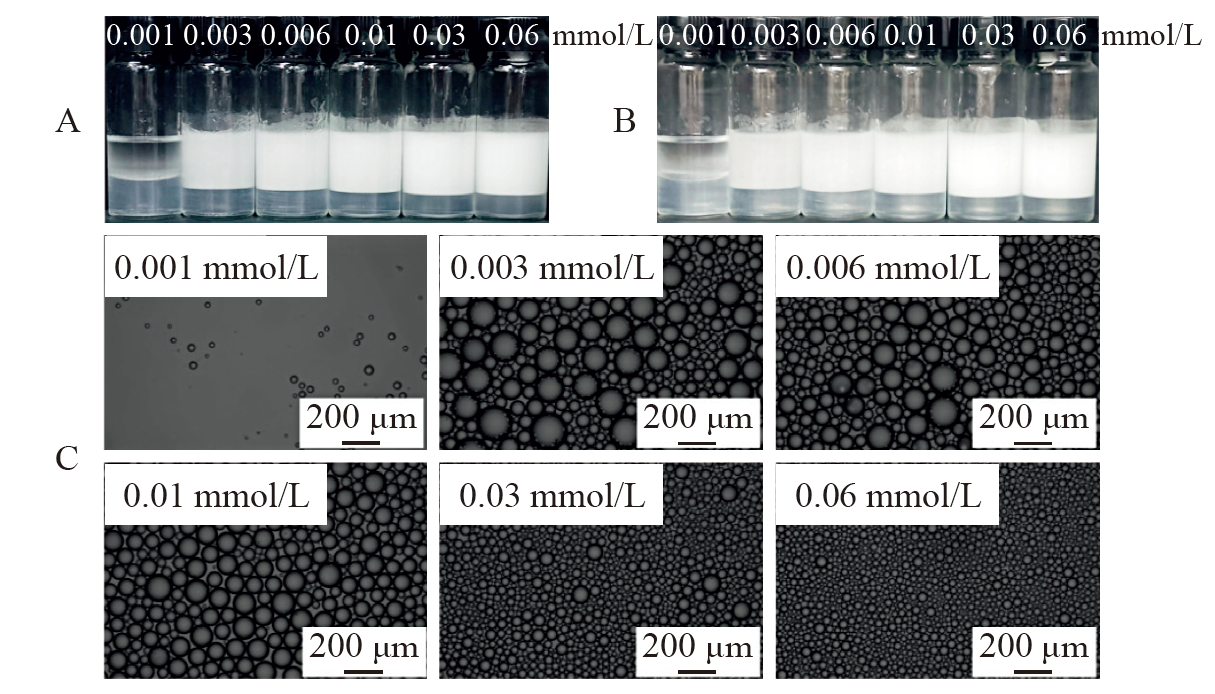

Fig. 13
(A) Photos and (B) optical micrographs of n-decane-in-water (7 mL/7 mL) Pickering emulsions co-stabilized by 0.1 wt.% silica nanoparticles and 0.1 mmol/L CH3(EO) 5-R11-COOH at pH of 3 undergoing switching off/on cycles induced by heating and cooling, taken 12 h after operation. After each demulsification the separated oil was removed and fresh oil was added for the next re-homogenization"
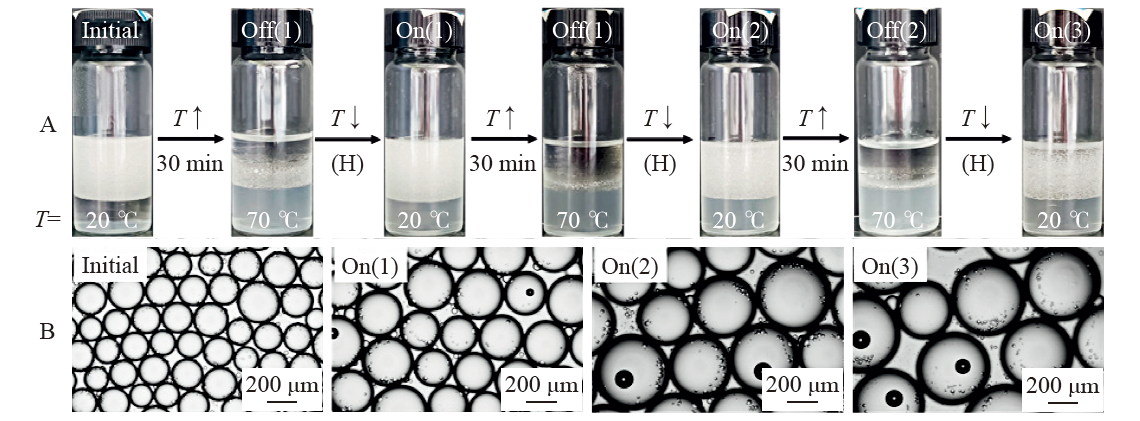
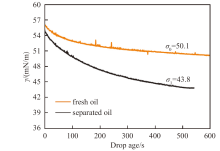
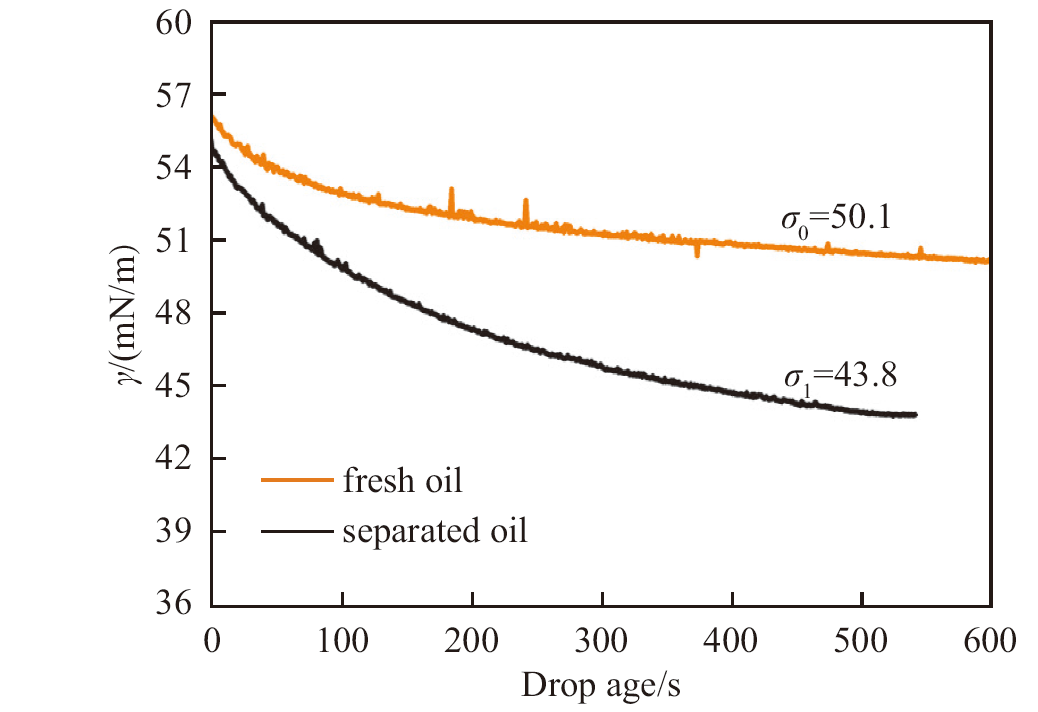
Fig. 19
Dynamic interfacial tension between n-decane and water measured by the drop shape method at 25 ℃. The equilibrium interfacial tension between fresh oil and pure water is 50.1 mN/m, but that between pure water and the oil separated from the equivolumetric mixture of oil and an aqueous solution of 1 mmol/L CH3O(EO) 5-R11-COONa at pH of 10.9 is much lower, suggesting the transfer of surfactant to the oil phase"
| [1] |
Tang J T, Quinlan P J, Tam K C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications[J]. Soft Matter, 2015, 11: 3512-3529.
doi: 10.1039/c5sm00247h pmid: 25864383 |
| [2] |
Malcolm A S, Dexter A F, Middelberg A P J. Foaming properties of a peptide designed to form stimuli-responsive interfacial films[J]. Soft Matter, 2006, 2: 1057-1066.
doi: 10.1039/b609960b pmid: 32680208 |
| [3] |
Balasuriya T S, Dagastine R R. Interaction forces between bubbles in the presence of novel responsive peptide surfactants[J]. Langmuir, 2012, 28: 17230-17237.
doi: 10.1021/la304351a pmid: 23181754 |
| [4] |
Dexter A F, Malcolm A S, Middelberg A P J. Reversible active switching of the mechanical properties of a peptide film at a fluid-fluid interface[J]. Nat. Mater., 2006, 5: 502-506.
pmid: 16715085 |
| [5] |
Liu Y, Jessop P G, Cunningham M C, et al. Switchable surfactants[J]. Science, 2006, 313: 958-960.
pmid: 16917059 |
| [6] |
Zhang Y, Feng Y, Wang J, et al. CO2-Switchable wormlike micelles[J]. Chem. Commun., 2013, 49: 4902-4904.
doi: 10.1039/c3cc41059e |
| [7] |
Jiang J Z, Zhu Y, Cui Z G, et al. Switchable Pickering emulsions stabilized by silica nanoparticles hydrophobized in situ with a switchable surfactant[J]. Angew. Chem. Int. Ed., 2013, 52: 12373-12376.
doi: 10.1002/anie.v52.47 |
| [8] |
Saji T, Hoshino K, Aoyagui S. Reversible formation and disruption of micelles by control of the redox state of the head group[J]. J. Am. Chem. Soc., 1985, 107: 6865-6868.
doi: 10.1021/ja00310a020 |
| [9] |
Aydogan N, Abbott N L. Comparison of the surface activity and bulk aggregation of ferrocenyl surfactants with cationic and anionic headgroups[J]. Langmuir, 2001, 17: 5703-5706.
doi: 10.1021/la010178e |
| [10] | Schmittel M, Lal M, Graf K, et al. N,N′-dimethyl-2, 3-dialkylpyrazinium salts as redox-switchable surfactants? Redox, spectral, EPR and surfactant properties[J]. Chem. Commun., 2005, 41: 5650-5652. |
| [11] |
Kang H C, Lee B M, Yoon J, et al. Synthesis and surface-active properties of new photosensitive surfactants containing the azobenzene group[J]. J. Colloid Interface Sci., 2000, 231: 255-264.
doi: 10.1006/jcis.2000.7158 |
| [12] |
Eastoe J, Dominguez M S, Wyatt P, et al. Properties of a stilbene-containing gemini photosurfactant: light-triggered changes in surface tension and aggregation[J]. Langmuir, 2002, 18: 7837-7844.
doi: 10.1021/la0257384 |
| [13] |
Chevallier E, Monteux C, Lequeux F, et al. Photofoams: Remote control of foam destabilization by exposure to light using an azobenzene surfactant[J]. Langmuir, 2012, 28: 2308-2312.
doi: 10.1021/la204200z pmid: 22280317 |
| [14] |
Li J, Zhao M, Zhou H, et al. Photo-induced transformation of wormlike micelles to spherical micelles in aqueous solution[J]. Soft Matter, 2012, 8: 7858-7864.
doi: 10.1039/c2sm25218j |
| [15] |
Raghavan S R, Edlund H, Kaler E W. Cloud-point phenomena in wormlike micellar systems containing cationic surfactant and salt[J]. Langmuir, 2002, 18: 1056-1064.
doi: 10.1021/la011148e |
| [16] |
Aathimanikandan S V, Savariar E N, Thayumanavan S. Temperature-sensitive dendritic micelles[J]. J. Am. Chem. Soc., 2005, 127: 14922-14929.
pmid: 16231948 |
| [17] |
Yang Z J, Wei J J, Sobolev Y I, et al. Systems of mechanized and reactive droplets powered by multi-responsive surfactants[J]. Nature, 2018, 553: 313-318.
doi: 10.1038/nature25137 |
| [18] |
Liang C, Harjani J R, Robert T, et al. Use of CO2-triggered switchable surfactants for the stabilization of oil-in-water emulsions[J]. Energy Fuels, 2012, 26: 488-494.
doi: 10.1021/ef200701g |
| [19] |
Fameau A L, Saint-Jalmes A, Cousin F, et al. Smart foams: switching reversibly between ultrastable and unstable foams[J]. Angew. Chem. Int. Ed., 2011, 50: 8264-8268.
doi: 10.1002/anie.v50.36 |
| [20] | Becher P. Encyclopedia of Emulsion Technology[M]. New York: Marcel Dekker, 1983. |
| [21] | Rosen M J, Kunjappu J T. Surfactants and Interfacial Phenomena (4th edition)[M]. Hoboken: John Wiley & Sons, 2012. |
| [22] | Israelachvili J N. Intermolecular and surface forces[M]. London: Academic Press, 1992. |
| [23] |
Binks B P. Particles as surfactants—similarities and differences[J]. Curr. Opin. Colloid Interface Sci., 2002, 7: 21-41.
doi: 10.1016/S1359-0294(02)00008-0 |
| [24] |
Aveyard R, Binks B P, Clint J H. Emulsions stabilised solely by colloidal particles[J]. Adv. Colloid Interface Sci., 2003, 100-102: 503-546.
doi: 10.1016/S0001-8686(02)00069-6 |
| [25] | Binks B P, Horozov T S. Colloid particles at liquid interfaces[M]. London: Cambridge University Press, 2006, Chapter 1. |
| [26] |
Xu M D, Jiang J Z, Pei X M, et al. Novel oil-in-water emulsions stabilized by ionic surfactant and similarly charged nanoparticles at very low concentrations[J]. Angew. Chem. Int. Ed., 2018, 130: 7864-7868.
doi: 10.1002/ange.v130.26 |
| [27] |
Xu M D, Xu L F, Lin Q, et al. Switchable oil-in-water emulsions stabilized by like-charged surfactants and particles at very low concentrations[J]. Langmuir, 2019, 35: 4058-4067.
doi: 10.1021/acs.langmuir.8b04159 pmid: 30807183 |
| [28] |
Lin Q, Xu M D, Cui Z G, et al. Structure and stabilization mechanism of diesel oil-in-water emulsions stabilized solely by either positively or negatively charged nanoparticles[J]. Colloids Surf. A, 2019, 573: 30-39.
doi: 10.1016/j.colsurfa.2019.04.046 |
| [29] |
Ravera F, Ferrari M, Liggieri L, et al. Liquid-liquid interfacial properties of mixed nanoparticle-surfactant systems[J]. Colloids Surf. A, 2008, 323: 99-108.
doi: 10.1016/j.colsurfa.2007.10.017 |
| [30] | Jiang J Z, Ma Y X, Cui Z G, et al. Pickering emulsions responsive to CO2/N2 and light dual stimuli at ambient temperature[J]. Langmuir, 2016, 32: 8868-8675. |
| [31] |
Xu M D, Zhang W Q, Pei X M, et al. CO2/N2 triggered switchable Pickering emulsions stabilized by alumina nanoparticles in combination with a conventional anionic surfactant[J]. RSC Adv., 2017, 7: 29742-29751.
doi: 10.1039/C7RA03722H |
| [32] |
Liu K H, Jiang J Z, Cui Z G, et al. pH-Responsive Pickering emulsions stabilized by silica nanoparticles in combination with a conventional zwitterionic surfactant[J]. Langmuir, 2017, 33: 2296-2305.
doi: 10.1021/acs.langmuir.6b04459 pmid: 28191963 |
| [33] |
Zhu Y, Jiang J Z, Liu K H, et al. Switchable Pickering emulsions stabilized by silica nanoparticles hydrophobized in situ with a conventional cationic surfactant[J]. Langmuir, 2015, 31: 3301-3307.
doi: 10.1021/acs.langmuir.5b00295 |
| [34] |
Zhu Y, Fu T, Liu K H, et al. Thermo-responsive Pickering emulsions stabilized by silica nanoparticles in combination with alkyl polyoxyethylene ether nonionic surfactant[J]. Langmuir, 2017, 33: 5724-5733.
doi: 10.1021/acs.langmuir.7b00273 |
| [35] |
Akartuna I, Studart A R, Tervoort E, et al. Stabilization of oil-in-water emulsions by colloidal particles modified with short amphiphiles[J]. Langmuir, 2008, 24: 7161-7168.
doi: 10.1021/la800478g pmid: 18547079 |
| [36] |
Wei Y, Tong Z, Dai L, et al. Novel colloidal particles and natural small molecular surfactants co-stabilized Pickering emulsions with hierarchical interfacial structure: Enhanced stability and controllable lipolysis[J]. J. Colloid Interface Sci., 2020, 563: 291-307.
doi: 10.1016/j.jcis.2019.12.085 |
| [37] |
Lan Q, Yang F, Zhang S, et al. Synergistic effect of silica nanoparticles and cetyltrimethyl ammonium bromide on the stabilization of O/W emulsions[J]. Colloids Surf. A, 2007, 302: 126-135.
doi: 10.1016/j.colsurfa.2007.02.010 |
| [38] |
Yu S J, Zhang H J, Jiang J Z, et al. Pickering emulsions of alumina nanoparticles and Bola-type selenium surfactant yield a fully recyclable aqueous phase[J]. Green Chem., 2020, 22: 5470-5475
doi: 10.1039/D0GC02016H |
| [39] |
Pei X M, Zhang S, Zhang W Q, et al. Behavior of smart surfactants in stabilizing pH-responsive emulsions[J]. Angew. Chem. Int. Ed., 2021, 60: 5235-5239.
doi: 10.1002/anie.202013443 pmid: 33258181 |
| [40] |
Liu P, Wu J H, Pei X M, et al. Recyclable surfactant containing dynamic covalent bond and relevant smart emulsions[J]. Green Chem., 2022, 24: 7612-7621.
doi: 10.1039/D2GC01977A |
| [41] |
Liu P, Zhang S, Pei X M, et al. Recyclable and re-usable smart surfactant for stabilization of various multi-responsive emulsions alone or with nanoparticles[J]. Soft Matter, 2022, 18: 849-858.
doi: 10.1039/d1sm01660a pmid: 34982810 |
| [42] |
Liu P, Pei X M, Cui Z G, et al. Recyclable nonionic-anionic bola surfactant as a stabilizer of size-controllable and pH-responsive Pickering emulsions[J]. Langmuir, 2023, 39: 841-850.
doi: 10.1021/acs.langmuir.2c02924 pmid: 36603129 |
| [43] | Attwood D, Florence A T. Surfactant systems, their chemistry, pharmacy and biology[M]. Chapman and Hall, 1983: 473. |
| [44] |
Cui Z G, Yang L L, Cui Y Z, et al. Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant[J]. Langmuir, 2010, 26: 4717-4724.
doi: 10.1021/la903589e pmid: 19950938 |
| [45] |
Cummins P G, Penfold J, Staples E. Nature of the adsorption of the nonionic surfactant pentaethylene glycol monododecyl ether on a ludox silica sol[J]. J. Phys. Chem., 1992, 96: 8092-8094.
doi: 10.1021/j100199a049 |
| [46] |
Zeng X, Osseo-Asare K. Partitioning behavior of silica in the PEG/dextran/H2O aqueous biphasic system[J]. Colloids and Surfaces A, 2003, 226: 45-54.
doi: 10.1016/S0927-7757(03)00354-6 |
| [47] |
Zhang L, Zhang G C, Ge J J, et al. pH- and thermo-responsive Pickering emulsion stabilized by silica nanoparticles and conventional nonionic copolymer surfactants[J]. J. Colloid Interface Sci., 2022, 616: 129-140.
doi: 10.1016/j.jcis.2022.02.067 |
| [48] |
Zhang R, Somasundaran P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces[J]. Adv. Colloid Interface. Sci., 2006, 123-126: 213-229.
doi: 10.1016/j.cis.2006.07.004 |
| [1] | Ding Zhengqing, Wu Yingyi, Wang Weiyun, Huang Xujuan, Cai Zhaosheng. Study on Pickering emulsion stabilized by hydroxyethyl cellulose/nanocellulose and its rheological properties [J]. China Surfactant Detergent & Cosmetics, 2023, 53(3): 245-252. |
| [2] | Ting Pan, Junhui Wu, Xiaomei Pei, Zhenggang Cui. Temperature and pH responsive behavior of wormlike micelles formed by novel pseudo-gemini surfactant [J]. China Surfactant Detergent & Cosmetics, 2023, 53(12): 1361-1368. |
| [3] | Chen Ningru, Zhang Ruoqi, Han Xu, Shang Yazhuo. New emulsion system and its application in cosmetics (III)Pickering emulsion [J]. China Surfactant Detergent & Cosmetics, 2023, 53(11): 1257-1265. |
| [4] | Shen Jiajun,Huan Jingjing,Wang Bijia,Sui Xiaofeng. Properties of oleogel-in-water Pickering emulsion stabilized by nano-chitin [J]. China Surfactant Detergent & Cosmetics, 2022, 52(8): 844-850. |
| [5] | Zhang Qianjie,Shen Xingliang,Sheng Taotao,Zhang Wanping,Xu Jianying. The interaction of cetyltrimethyl ammonium bromide-pearl powder and its stabilization of the double phase inversion in emulsions [J]. China Surfactant Detergent & Cosmetics, 2022, 52(5): 468-475. |
| [6] | Xie Xin,Wang Weihao,Liu Huanyu,Sun Mengmeng,Li Qinyuan,Jia Lufan,Meng Tao. Study on the Pickering emulsion stabilized by Alg@TiO2 microspheres for sunscreen formulation [J]. China Surfactant Detergent & Cosmetics, 2022, 52(3): 229-236. |
| [7] | Chen Yunbo,Li Xinyi,Mao Zhiping,Xu Hong,Sui Xiaofeng. Fabrication of phase change microcapsules via nano-chitin stabilized Pickering emulsion [J]. China Surfactant Detergent & Cosmetics, 2022, 52(12): 1286-1292. |
| [8] | Huan Jingjing,Wang Bijia,Mao Zhiping,Sui Xiaofeng. Rapid preparation of nano-chitin for stabilizing Pickering emulsions [J]. China Surfactant Detergent & Cosmetics, 2022, 52(10): 1081-1087. |
| [9] | Shen Yongqiang,Sun Yajuan,Yang Cheng,Wang Jing. Study on the preparation and emulsifying properties of peach seed protein isolate nanoparticles [J]. China Surfactant Detergent & Cosmetics, 2021, 51(9): 809-816. |
| [10] | Yu Hui,Zhu Yongfeng,Hui Aiping,Yang Fangfang,Wang Aiqin. Advances in the application of attapulgite in Pickering emulsion preparation [J]. China Surfactant Detergent & Cosmetics, 2021, 51(7): 670-678. |
| [11] | GONG Cheng-yi,YU Hao,WANG Qi-qi,SUN Ji-yong,SONG Ai-xin. Progress on stimulus-responsive Pickering emulsions and their applications [J]. China Surfactant Detergent & Cosmetics, 2021, 51(6): 554-563. |
| [12] | FANG Zhen-xing,WANG Xi-ying,WU Jing,ZENG Ying,PAN Hong,XIE Zhen-hua. Preparation of Pickering emulsion from volcano mud and its pH stability [J]. China Surfactant Detergent & Cosmetics, 2021, 51(6): 506-512. |
| [13] | HE Yi-jing,XU Hu-jun. Preparation of Pickering emulsion stabilized by lauroyl lysine [J]. China Surfactant Detergent & Cosmetics, 2021, 51(5): 413-420. |
| [14] | CHEN Feng-feng,TAO Sheng-nan,GONG Sui-jing,ZHANG Sheng-wei,SUN Ya-juan,YANG Cheng,LI Yun-xing. Cosmetic emulsions and new technologies of emulsification (I) Fundamental principles of Pickering emulsions and their applications in cosmetics [J]. China Surfactant Detergent & Cosmetics, 2021, 51(2): 89-97. |
| [15] | Hu Jiawen,Fan Ye,Fang Yun,Wang Hong. Aqueous two-phase Pickering emulsions stabilized by the CLAA@CaCO3 nanoparticles [J]. China Surfactant Detergent & Cosmetics, 2021, 51(12): 1186-1191. |
|