


China Surfactant Detergent & Cosmetics ›› 2022, Vol. 52 ›› Issue (12): 1366-1372.doi: 10.3969/j.issn.1001-1803.2022.12.016
• Reviews • Previous Articles
Received:2022-01-04
Revised:2022-12-01
Online:2022-12-22
Published:2022-12-29
Contact:
Gaoning Fan
E-mail:gn_fan@163.com.
CLC Number:
Fan Gaoning. Research progress in cosmetics applications and properties of mycosporine-like amino acids[J].China Surfactant Detergent & Cosmetics, 2022, 52(12): 1366-1372.
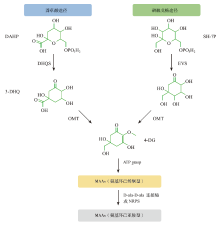
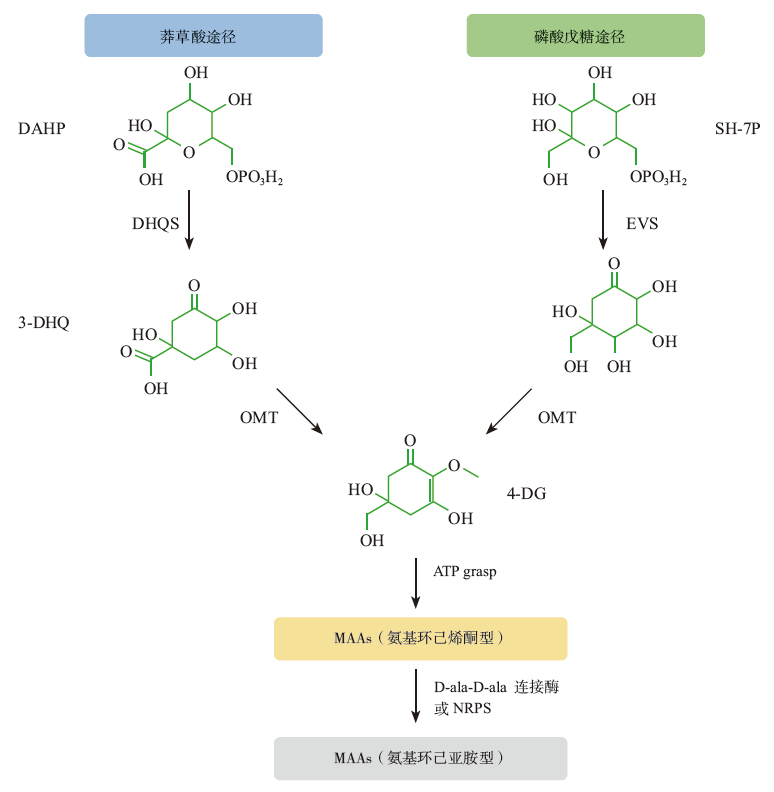
Fig. 2
Proposed biosynthetic pathways of mycosporine-like amino acids (DAHP: 3-deoxy-D-arabino-heptulosonate phosphate; DHQS: 3-dehydroquinate synthase; 3-DHQ: 3-dehydroquinate; SH-7P: sedoheptulose-7-phosphate; EVS: cyclase-2-epi-5-epi-valiolone synthase; OMT: O-methyltransferase; 4-DG: 4-deoxygadusol; ATP: adenosine triphosphate; NRPS: nonribosomal peptide synthase; D-ala-D-ala-ligase: D-alanyl-D-alanine-ligase)"
| [1] |
Stoddard M, Lyons A, Moy R. Skin cancer prevention: A review of current oral options complementary to sunscreens[J]. J Drugs Dermatol, 2018, 17 (12) : 1266-1271.
doi: S1545961618P1266X pmid: 30586257 |
| [2] |
de la Coba F, Aguilera J, Korbee N, et al. UVA and UVB photoprotective capabilities of topical formulations containing mycosporine-like amino acids (MAAs) through different biological effective protection factors[J]. Mar Drugs, 2019, 17 (1) : 55.
doi: 10.3390/md17010055 |
| [3] |
Geraldes V, Pinto E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features[J]. Pharmaceuticals (Basel), 2021, 14 (1) : 63.
doi: 10.3390/ph14010063 |
| [4] |
Sun Y, Zhang N, Zhou J, et al. Distribution, contents, and types of mycosporine-like amino acids (MAAs) in marine macroalgae and a database for MAAs based on these characteristics[J]. Mar Drugs, 2020, 18 (1) : 43.
doi: 10.3390/md18010043 |
| [5] |
Shick J M, Dunlap W C. Mycosporine-like amino acids and related gadusols: Biosynthesis, acumulation, and UV-protective functions in aquatic organisms[J]. Annu Rev Physiol, 2002, 64: 223-262.
pmid: 11826269 |
| [6] |
Carreto J I, Carignan M O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects[J]. Mar Drugs, 2011, 9 (3) : 387-446.
doi: 10.3390/md9030387 pmid: 21556168 |
| [7] |
Ishihara K, Watanabe R, Uchida H, et al. Novel glycosylated mycosporine-like amino acid, 13-o-(β-galactosyl)-porphyra-334, from the edible cyanobacterium nostoc sphaericum-protective activity on human keratinocytes from UV light[J]. J Photochem Photobiol B, 2017, 172: 102-108.
doi: 10.1016/j.jphotobiol.2017.05.019 |
| [8] |
Lawrence K P, Long P F, Young A R. Mycosporine-like amino acids for skin photoprotection[J]. Curr Med Chem, 2018, 25 (40) : 5512-5527.
doi: 10.2174/0929867324666170529124237 pmid: 28554325 |
| [9] | La Barre S, Roullier C, Boustie J. Mycosporine-like amino acids (MAAs) in biological photosystems[M]. USA: Wiley-VCH Verlag GmbH & Co. KGaA, 2014: 333-360. |
| [10] | Singh A, Čížková M, Bišová K, et al. Exploring mycosporine-like amino acids (MAAs) as safe and natural protective agents against UV-induced skin damage[J]. Antioxidants (Basel), 2021, 10 (5) : 683. |
| [11] |
Álvarez-Gómez F, Korbee N, Casas-Arrojo V, et al. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts[J]. Molecules, 2019, 24 (2) : 341.
doi: 10.3390/molecules24020341 |
| [12] |
Oyamada C, Kaneniwa M, Ebitani K, et al. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells[J]. Mar Biotechnol (NY), 2008, 10 (2) : 141-150.
doi: 10.1007/s10126-007-9043-z |
| [13] |
Ryu J, Park S J, Kim I H, et al. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts[J]. Int J Mol Med, 2014, 34 (3) : 796-803.
doi: 10.3892/ijmm.2014.1815 pmid: 24946848 |
| [14] |
Pope M A, Spence E, Seralvo V, et al. O-methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413[J]. Chembiochem, 2015, 16 (2) : 320-327.
doi: 10.1002/cbic.201402516 |
| [15] |
Balskus E P, Walsh C T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria[J]. Science, 2010, 329 (5999) : 1653-1656.
doi: 10.1126/science.1193637 pmid: 20813918 |
| [16] |
Katoch M, Mazmouz R, Chau R, et al. Heterologous production of cyanobacterial mycosporine-like amino acids mycosporine-ornithine and mycosporine-lysine in Escherichia coli[J]. Appl Environ Microbiol, 2016, 82 (20) : 6167-6173.
doi: 10.1128/AEM.01632-16 |
| [17] |
Geraldes V, Medeiros L S d, Lima S T, et al. Genetic and biochemical evidence for redundant pathways leading to mycosporine-like amino acid biosynthesis in the cyanobacterium[J]. Algae, 2020, 35 (2) : 177-187.
doi: 10.4490/algae.2020.35.5.19 |
| [18] |
Shick J M, Romaine-Lioud S, Romaine-Lioud S, et al. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral stylophora pistillata despite decreases in its population of symbiotic dinoflagellates[J]. Limnology and Oceanography, 1999, 44 (7) : 1667-1682.
doi: 10.4319/lo.1999.44.7.1667 |
| [19] |
Spence E, Dunlap W C, Shick J M, et al. Redundant pathways of sunscreen biosynthesis in a cyanobacterium[J]. ChemBioChem, 2012, 13 (4) : 531-533.
doi: 10.1002/cbic.201100737 pmid: 22278966 |
| [20] | Wada N, Sakamoto T, Matsugo S. Mycosporine-like amino acids and their derivatives as natural antioxidants[J]. Antioxidants (Basel), 2015, 4 (3) : 603-646. |
| [21] |
Fernandes S C, Alonso-Varona A, Palomares T, et al. Exploiting mycosporines as natural molecular sunscreens for the fabrication of UV-absorbing green materials[J]. ACS Appl Mater Interfaces, 2015, 7 (30) : 16558-16564.
doi: 10.1021/acsami.5b04064 |
| [22] |
Conde F R, Churio M S, Previtali C M. Experimental study of the excited-state properties and photostability of the mycosporine-like amino acid palythine in aqueous solution[J]. Photochem Photobiol Sci, 2007, 6 (6) : 669-674.
doi: 10.1039/b618314j |
| [23] |
Conde F R, Churio M S, Previtali C M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution[J]. Photochem Photobiol Sci, 2004, 3 (10) : 960-967.
doi: 10.1039/b405782a |
| [24] |
Conde F R, Churio M S, Previtali C M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution[J]. J Photochem Photobiol B, 2000, 56 (2/3) : 139-144.
doi: 10.1016/S1011-1344(00)00066-X |
| [25] |
Whitehead K, Hedges J I. Photodegradation and photosensitization of mycosporine-like amino acids[J]. J Photochem Photobiol B, 2005, 80 (2) : 115-121.
doi: 10.1016/j.jphotobiol.2005.03.008 |
| [26] |
Torregiani J H, Lesser M P. The effects of short-term exposures to ultraviolet radiation in the Hawaiian Coral Montipora verrucosa[J]. Journal of Experimental Marine Biology and Ecology, 2007, 340 (2) : 194-203.
doi: 10.1016/j.jembe.2006.09.004 |
| [27] |
Misonou T, Saitoh J, Oshiba S, et al. UV-absorbing substance in the red alga Porphyra yezoensis (bangiales, rhodophyta) block thymine photodimer production[J]. Mar Biotechnol (NY), 2003, 5 (2) : 194-200.
doi: 10.1007/s10126-002-0065-2 |
| [28] |
McAdam E, Brem R, Karran P. Oxidative stress-induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy[J]. Mol Cancer Res, 2016, 14 (7) : 612-622.
doi: 10.1158/1541-7786.MCR-16-0053 pmid: 27106867 |
| [29] |
Rosic N N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens[J]. Mar Drugs, 2019, 17 (11) : 638.
doi: 10.3390/md17110638 |
| [30] |
Fuentes-Tristan S, Parra-Saldivar R, Iqbal H M N, et al. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. With UV-protection potentialities[J]. J Photochem Photobiol B, 2019, 201: 111684.
doi: 10.1016/j.jphotobiol.2019.111684 |
| [31] |
de la Coba F, Aguilera J, Figueroa F L, et al. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen[J]. Journal of Applied Phycology, 2009, 21 (2) : 161-169.
doi: 10.1007/s10811-008-9345-1 |
| [32] |
Lawrence K P, Gacesa R, Long P F, et al. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine[J]. Br J Dermatol, 2018, 178 (6) : 1353-1363.
doi: 10.1111/bjd.16125 pmid: 29131317 |
| [33] |
Rastogi R P, Sonani R R, Madamwar D, et al. Characterization and antioxidant functions of mycosporine-like amino acids in the Cyanobacterium nostoc sp. R76dm[J]. Algal Research, 2016, 16: 110-118.
doi: 10.1016/j.algal.2016.03.009 |
| [34] |
Tarasuntisuk S, Palaga T, Kageyama H, et al. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated raw 264.7 macrophages[J]. Archives of Biochemistry and Biophysics, 2019, 662: 33-39.
doi: S0003-9861(18)30711-2 pmid: 30502329 |
| [35] | Kim S Y, Cho W K, Kim H I, et al. Transcriptome profiling of human follicle dermal papilla cells in response to porphyra-334 treatment by rna-seq[J]. Evid Based Complement Alternat Med, 2021: 6637513. |
| [36] |
Suh S S, Hwang J, Park M, et al. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity[J]. Mar Drugs, 2014, 12 (10) : 5174-5187.
doi: 10.3390/md12105174 |
| [37] |
Rui Y, Zhaohui Z, Wenshan S, et al. Protective effect of MAAs extracted from porphyra tenera against UV irradiation-induced photoaging in mouse skin[J]. J Photochem Photobiol B, 2019, 192: 26-33.
doi: 10.1016/j.jphotobiol.2018.12.009 |
| [38] | Mibelle Group Biochemistry. Helioguard 365 a natural UV a-screening compound from sea algae to protect the skin against photo-aging[EB/OL]. [2022-01-04]. https://www.xmedicimports.com/shop/images/Helioguard-365.pdf. |
| [39] |
Fernando C, Martin M, Diego L. UV sunscreens of microbial origin: Mycosporines and mycosporine- like aminoacids[J]. Recent Patents on Biotechnology, 2014, 8 (3) : 179-193.
pmid: 25619303 |
| [40] |
Chrapusta E, Kaminski A, Duchnik K, et al. Mycosporine-like amino acids: Potential health and beauty ingredients[J]. Mar Drugs, 2017, 15 (10) : 326.
doi: 10.3390/md15100326 |
| [41] |
Losantos R, Funes-Ardoiz I, Aguilera J, et al. Rational design and synthesis of efficient sunscreens to boost the solar protection factor[J]. Angew Chem Int Ed Engl, 2017, 56 (10) : 2632-2635.
doi: 10.1002/anie.201611627 |
| [1] | Kuankuan Gao, Suzhen Yang, Tingting Han, Yan Li, Chunying Yuan, Xinyu Mao. Research progress of royal jelly acid and its skin care efficacy [J]. China Surfactant Detergent & Cosmetics, 2024, 54(2): 209-215. |
| [2] | Meng Xianyao, Cheng Yidan, Guo Miaomiao, Ling Xiao, Yu Dan, Li Li. Research and application of Tibetan characteristic plant resources in cosmetics [J]. China Surfactant Detergent & Cosmetics, 2023, 53(6): 698-705. |
| [3] | Xiaoman Sun, Xianyao Meng, Wen Zhou, Miaomiao Guo, Xiao Ling, Li Li. Research and application of Yunnan characteristic plant resources in cosmetics [J]. China Surfactant Detergent & Cosmetics, 2023, 53(12): 1459-1465. |
| [4] | Wang Changyun,Hao Yang,Fan Jianru,Tang Wenjun,Xu Guiyun,Fan Jinshi. Preparation, properties and applications of natural biomass materials (Ⅵ) Straight or branched energy storage polysaccharide from plants: starch [J]. China Surfactant Detergent & Cosmetics, 2022, 52(6): 585-593. |
| [5] | LIU Xiao-qing,LIU Yu-hang,CHEN Yu-yan,JIANG Li-gang. Properties of polyglycerol fatty acid esters and its applications in cosmetics [J]. China Surfactant Detergent & Cosmetics, 2020, 50(2): 118-123. |
| [6] | LI Xin-en, HE Qiu-xing, OU Zi-cong, FANG Dian-li, GUAN Jian-yun, ZHAO Hong. Antimicrobial effectiveness evaluation of natural microorganism source preservatives used for cosmetic formulation [J]. China Surfactant Detergent & Cosmetics, 2015, 45(11): 639-642. |
| [7] | ZHANG Yan-ting, LI Xiao-feng, JIA Miao-juan, JING Chao, SUN Xiao-tao, DOU Tao. Synthesis of EU-1 zeolite without use of sodium hydroxide and its catalytic performance [J]. China Surfactant Detergent & Cosmetics, 2014, 44(7): 387-389. |
|
